SAAD Essay Prizes: Guidelines for Authors
Contribution formats
Essays in all categories are only accepted in digital format and via email. It is a condition of acceptance that they are the work solely of the author stated and that they have not been previously published elsewhere (either in print or electronic format) nor are they under consideration for publication by any other periodical.
Essays should meet the following criteria: they should be original, clearly written, relevant to dentistry, and may be on any subject related to conscious sedation, anxiety control, general anaesthesia or analgesia in dentistry and designed to inform, add to discussion or debate, or entertain. In addition, research papers should have appropriate study methods, valid data and conclusions that are supported by the data.
The authorship of the essay can only be attributed to a single author.
If in any doubt remains about the format or content of a proposed essay please contact the Executive Secretary before submission, prizes@saad.org.uk
Generative Artificial Intelligence (AI)
Generative artificial intelligence (AI) may not be used to write your essay. Some examples of generative AI products include ChatGPT, Microsoft CoPilot and Google Bard.
Submission
Essays may only be submitted to SAAD at prizes@saad.org.uk to arrive and be acknowledged before or on the deadline of 31st March each year.
Authors should note that submitted essays not fully conforming to these ‘Authors Guidelines’, especially in terms of length and manuscript format, will be returned for correction and may well be delayed or subsequently declined.
Length of contributions
Essays should be within the word count for the prize for which they are submitted:
- Drummond-Jackson Prize – 5000
- Undergraduate Essay prize – 3000
Titles must be descriptive of the contents of the article, but should be concise.
Essays should be introduced with a short abstract that should be able to stand alone. The abstract should not contain references or abbreviations, and should be no longer than 200 words. The abstract will not contribute to the word limit of the essay.
Manuscript Format
Manuscripts should be word-processed in Microsoft Word in the native format, single column and double-spaced with a margin of at least 4 cm on the left-hand side. No decorative borders should be used. The font used should be 12 point Calibri.
The pages should be numbered consecutively with the numbers at the bottom of each page.
The first page of the essay should give only the title of the article, and the author’s name, qualifications and work address (or Dental School address for students), including email address, and the prize for which the essay is submitted.
The layout of the text should be kept as simple as possible. Most formatting codes will be removed and replaced on processing the essay. In particular, do not use the word processor's options to justify text or to hyphenate words. However, do use bold face, italics, subscripts, superscripts etc.
To avoid unnecessary errors, you are strongly advised to use the 'spell-check' and 'grammar-check' functions of your word processor.
Data or tables may be submitted in Microsoft Excel format or embedded in the text of the Word document.
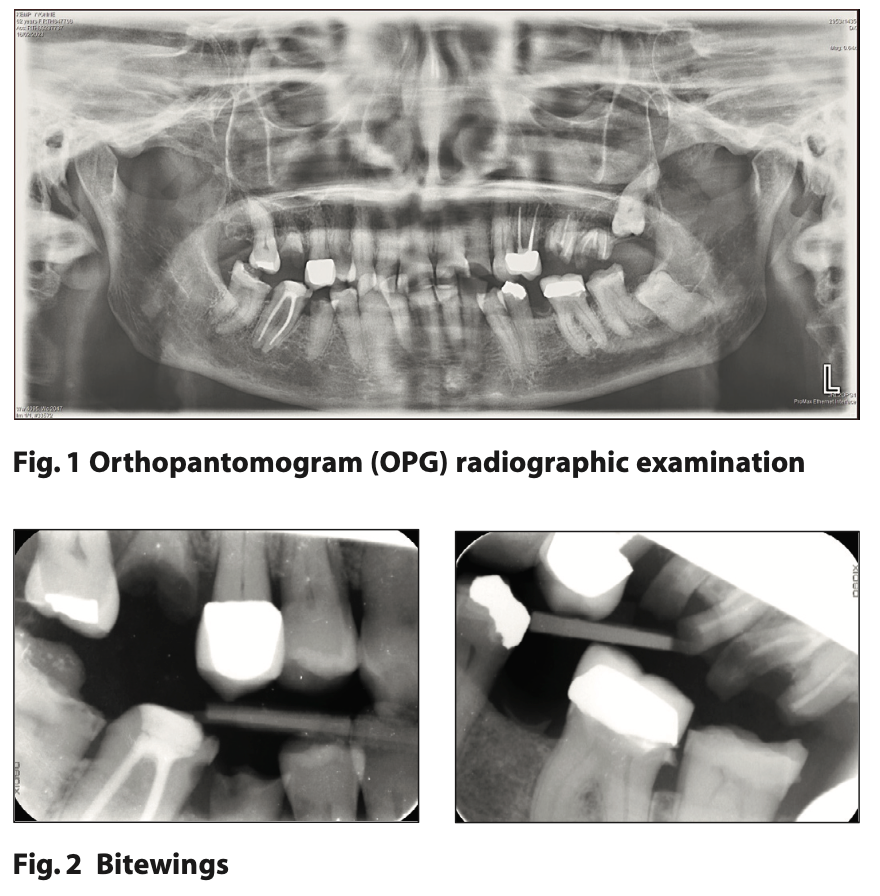
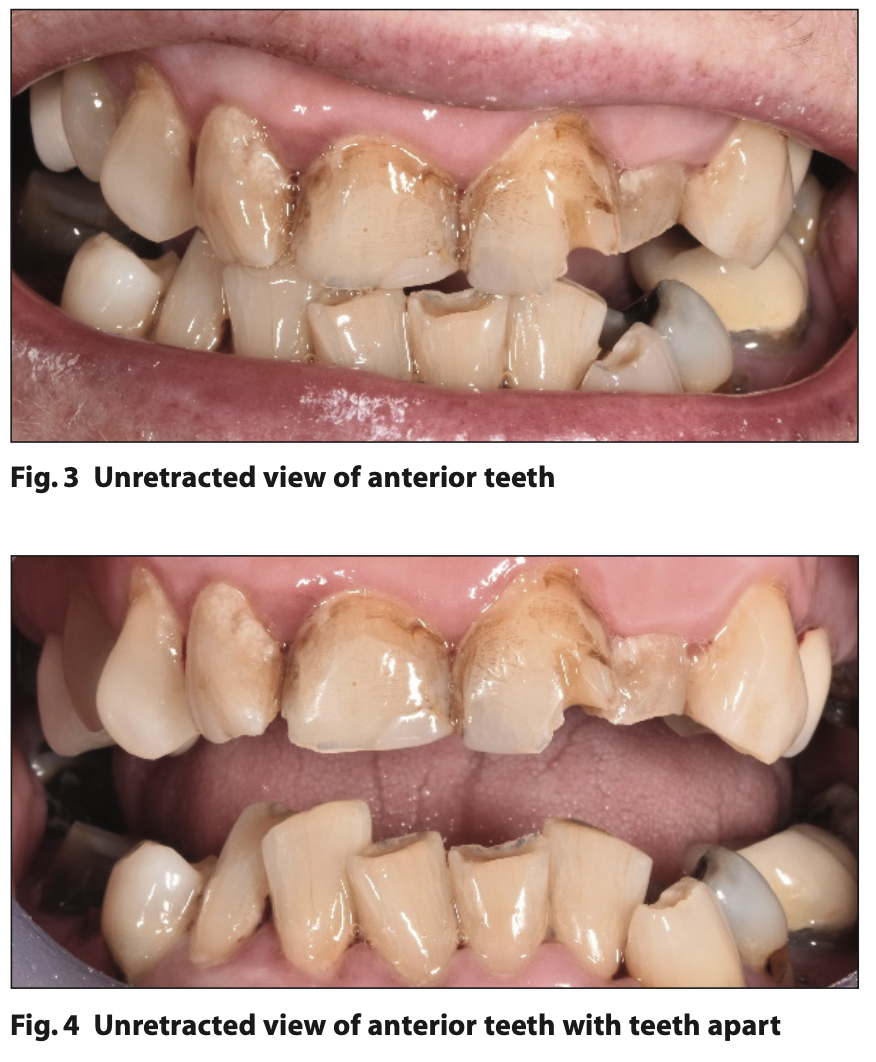
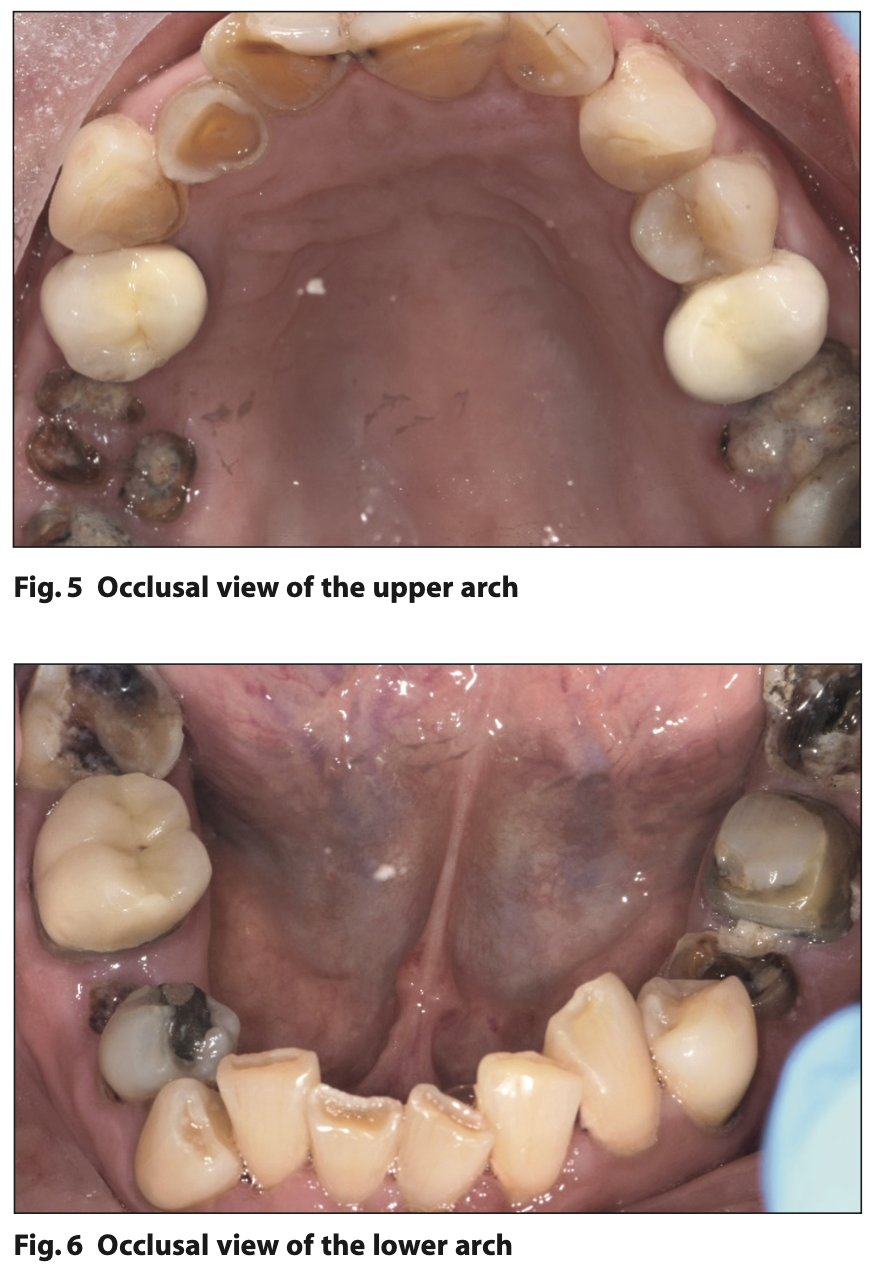
Figures or images should be submitted as separately attached and clearly labelled files in JPEG format at a high resolution of 300 dpi. Colour illustrations are preferred where possible. If the illustration is of a subject’s face, written consent for its publication must be obtained from the subject and attached with the essay. Should the essay be accepted for publication in the SAAD Digest, illustrations obtained from other sources such as books, or from colleagues, must again be accompanied by appropriate documentation indicating approval for their publication as part of the essay from the copyright holder, or individual concerned.
Units used in the manuscript must conform to the Système Internationale d’Unités (SI).
References
References must be in the Vancouver style, as used in the SAAD Digest (see below) They should be numbered in the order in which they appear in the text. The numbers should be inserted as superscripts each time the author is cited (Robb3-5 reported similar findings). Other references to the paper should be given in the same way after punctuation (Other studies have shown this to be true.4,5 Drummond-Jackson et al.6 demonstrated...)
At the end of the article the full list of references should give the names and initials of all authors unless there are more than six, in which case only the first three should be given followed by et al. The authors' names must be followed by the title
of the article; the title of the journal abbreviated according to Index Medicus and Index to Dental Literature style; year of publication; volume number; and the first and last page numbers in full. Titles of books should be followed by the place of publication, publisher, and the year. If this reference citation style is not followed exactly, especially in relation to punctuation and spacing, the manuscript will be returned without review.
Examples of reference styles (Note: The issue number of a Journal is not cited)
Reference to an article
1. Molar L R, Fang-Jones Q, Jaw U. Are teeth biting back?. Br Dent J 2006; 200: 144-146.
Reference to a book
2. Craig D C, Skelly A M. Practical Conscious Sedation. 1st ed. London: Quintessence, 2004.
Reference to a book chapter
3. Robb N D. Conscious s
Sedation in Dentistry. In Heasman PA (ed) Master Dentistry. Vol. 2; Restorative Dentistry, Paediatric Dentistry and Orthodontics. pp 149-168. Edinburgh: Churchill Livingstone, 2003.
Reference to a report
4. Re-accreditation and re-certification for the dental profession. London: General Dental Council, 1997.
Reference to a webpage
3. General Dental Council. Scope of practice. 2009. Online information available at www.gdc- uk.org/Newsandpublications/Publications/Publications/ScopeofpracticeApril20 09[1].pdf (accessed April 2012).
The author/principal author is responsible for the accuracy of the reference list. Acknowledgements
These should be grouped in a paragraph at the end of the text before the references. Permission and approval of the wording must be obtained from the person(s) thanked. Where any research project was supported by industry, this should be acknowledged in a covering letter to the Executive Secretary on submission of the essay.
Declaration of interests
Authors must ensure that they declare any possible conflicts of interest in their Essay. This includes matters such as: direct funding from an organisation or company for the research; funding received (or payment in kind) for any related work carried out from an organisation or company that could be linked to the research; consultation or advisory positions held in an organisation or company involved in the research or an organisation involved in similar research; any other situation that could be construed as a conflict of interest.
Ethics
Essays reporting clinical research must include a statement indicating that appropriate Ethical Committee approval has been granted.
SAAD Digest
Any essay submitted for a SAAD Essay prize may be considered for publication in the SAAD Digest.
The SAAD Digest is the Journal of the Society for the Advancement of Anaesthesia in Dentistry and has been published regularly in London UK, since 1970. It has been produced in its current format since 2006. Two editions are published each year. Copies of all editions produced since then are available online at http://www.saad.org.uk/index.php/digest-newsletters
The Digest has become a unique and invaluable international forum for all interested in advancement of knowledge in pain and anxiety control for dentistry.
Copyright
Upon acceptance for publication in SAAD Digest, it is assumed that the author assigns copyright of the essay to the Society for the Advancement of Anaesthesia in Dentistry. Single copies of the published essay for personal study may be made free of charge but multiple copies will require permission of the Editor prior to production.
Reviewed: February 2024