
Please click on the tables and figures to enlarge
Remimazolam compared to midazolam for dental sedation: an umbrella review
G. Shaw*1 BDS MSc (Oral Surgery) MFDS RCPS (Glasg)
K. Taylor2 PhD FDS.RCS (Oral Surgery) FDS.RCS (Eng. and Ed.) BDS BSc (Hons) Dip Con Sed
1Dental Surgeon, The Albion Clinic, Glasgow, G1 1RU
2Professor of Oral Surgery, University of Central Lancashire, Preston, Lancashire, PR1 2HE
Correspondence to: Graeme.shaw1@nhs.scot
Shaw G, Taylor K. Remimazolam compared to midazolam for dental sedation: an umbrella review. SAAD Dig. 2024: 40(1): 3-8
Abstract
Remimazolam is a newly approved benzodiazepine drug used for intravenous sedation. Its efficacy and safety compared to the standard sedative drug, midazolam, have not been studied extensively, particularly in the dental setting. This study aims to compare the outcomes of remimazolam and midazolam for single-drug intravenous sedation and to discuss its potential use in dentistry.
A search was conducted across six electronic databases for systematic reviews comparing the efficacy and safety of remimazolam and midazolam. Five systematic reviews were included from a total of 542 studies. The findings indicated that remimazolam may offer significant advantages over midazolam, including faster onset, higher procedure success rates, reduced need for rescue sedatives, shorter recovery time, improved cognitive recovery, and fewer instances of hypoxia. However, there were no significant findings regarding procedure completion or required sedation dosage.
Overall, the evidence suggests that remimazolam has statistically significant benefits over midazolam for intravenous sedation. However, more clinical trials are needed to determine its suitability and clinical significance in dental practice. Further research is required to fully understand the potential advantages of remimazolam in the dental setting.
Introduction
Remimazolam is a promising benzodiazepine drug that offers potential advantages over midazolam for procedural sedation.1 With a similar structure and mode of action to midazolam, remimazolam claims to offer a faster onset, shorter duration of action, and faster recovery from sedation.2,3 It has a significantly shorter distribution half-life and terminal elimination half-life, resulting in a quicker recovery.3 Remimazolam demonstrates a comparable safety profile to midazolam, without an increased risk of respiratory depression, cardiovascular complications, or prolonged sedation.4 Additionally, it can be reversed using flumazenil, similar to midazolam.5
Prolonged recovery from sedation with midazolam poses logistical challenges for clinics and patients, requiring escorts and extended supervision.6 If remimazolam allows for shorter recovery times and earlier discharge, it could increase patient throughput and convenience of intravenous sedation.
Phase III trials have been completed, and regulatory approval has been obtained for remimazolam in the EU and the UK.10 While various trials have explored remimazolam's use in colonoscopy, gastroscopy, and bronchoscopy3 there is limited research on its potential use for dental procedures. In July 2022 the Scottish Medicines Consortium assessed remimazolam for use in NHS Scotland for colonoscopy and bronchoscopy procedures.11 This review found that remimazolam does indeed have certain advantages over midazolam, however, it was not approved for use within NHS Scotland due to the financial implications of using remimazolam compared to the more affordable midazolam. This does not, however, mean that it cannot be used in independent or private healthcare settings, which would include the majority of dental practices.
The IACSD (Intercollegiate Advisory Committee on Sedation in Dentistry) released a statement indicating that practitioners experienced in midazolam use do not need additional supervised practice to administer remimazolam. They must familarise themselves with the pharmacology, dosing and indications together with an understanding of how these fit with the IACSD standards, which can be via CPD courses with appropriate aims and objectives.7 However, it outlines that until more substantial evidence becomes available, remimazolam should be used similarly to midazolam, with comparable requirements for escorts and restrictions on use in patients under 18.
If remimazolam proves to be as safe and effective as midazolam with faster onset and recovery, it could offer an improved alternative for ambulatory dental surgery.8 Clinical trials suggest that remimazolam has a comparable safety profile to midazolam, with lower incidence of respiratory depression and hypotension.9 However, further research specifically focused on its dental application is needed. This umbrella review aims to comprehensively assess the evidence, identify gaps, and evaluate the efficacy and safety of remimazolam compared to midazolam in dental sedation.
Method
Eligibility criteria
The eligibility criteria for inclusion in this study consisted of systematic reviews directly comparing remimazolam to midazolam in patients receiving intravenous procedural sedation, with, or without, meta-analysis. Studies that compared remimazolam to midazolam as well as placebo or other sedatives such as propofol were also included.
Excluded from the study were articles published as abstracts, editorials, letters, notes, opinions, posters, conference articles, methods, protocols or articles with unavailable full texts. There was no time restriction placed on the included articles due to the recent development and introduction of remimazolam. Duplicate publications and articles in languages other than English were also excluded.
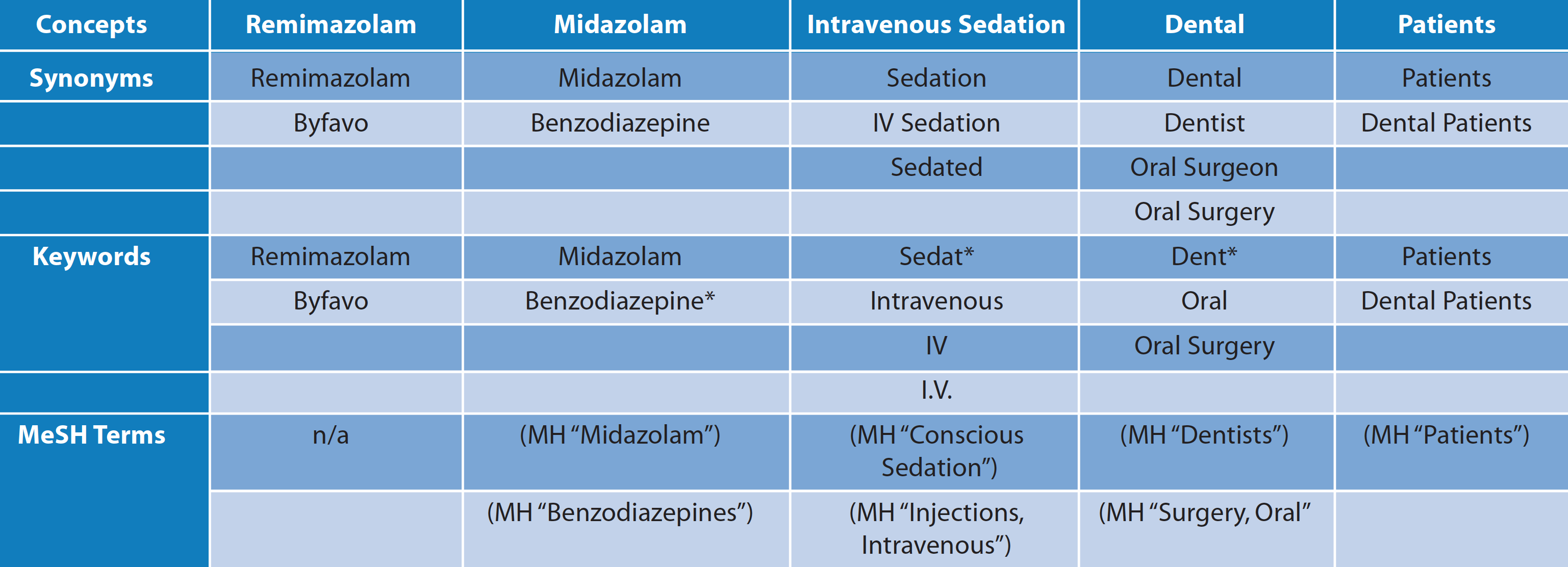 Table 1 Adapted Medline search strategy
Table 1 Adapted Medline search strategy
Additionally, studies focusing solely on remimazolam when used in conjunction with other sedatives, such as propofol, were excluded. This decision was based on the fact that polypharmaceutical sedation is not standard practice in the dental setting in the United Kingdom,6 making these studies non-generalisable and their results inapplicable to current practice in the UK. Some primary studies used fentanyl as an adjuvant opioid anaesthetic,12,15 these were not excluded as it was being used for intra-operative analgesia rather than the induction of sedation.12
Search strategy
A search strategy was generated and adapted as required for different databases. This strategy was adapted for use in the following databases: Medline, Dentistry and Oral Sciences Source, the Cumulative Index to Nursing & Allied Health, Academic Search Complete, Embase, and the Cochrane Library of Systematic Reviews. The reference lists of all included systematic reviews were handsearched to find any studies that may have been missed by the database search. Furthermore, PAION (the manufacturer of remimazolam) was contacted and any relevant studies were requested.
Results
Systematic review selection
Five studies were ultimately included in this review after full text analysis, assessment of the inclusion and exclusion criteria and evaluation of their relevance to the research question. The process of study selection has been summarised in Figure 1.
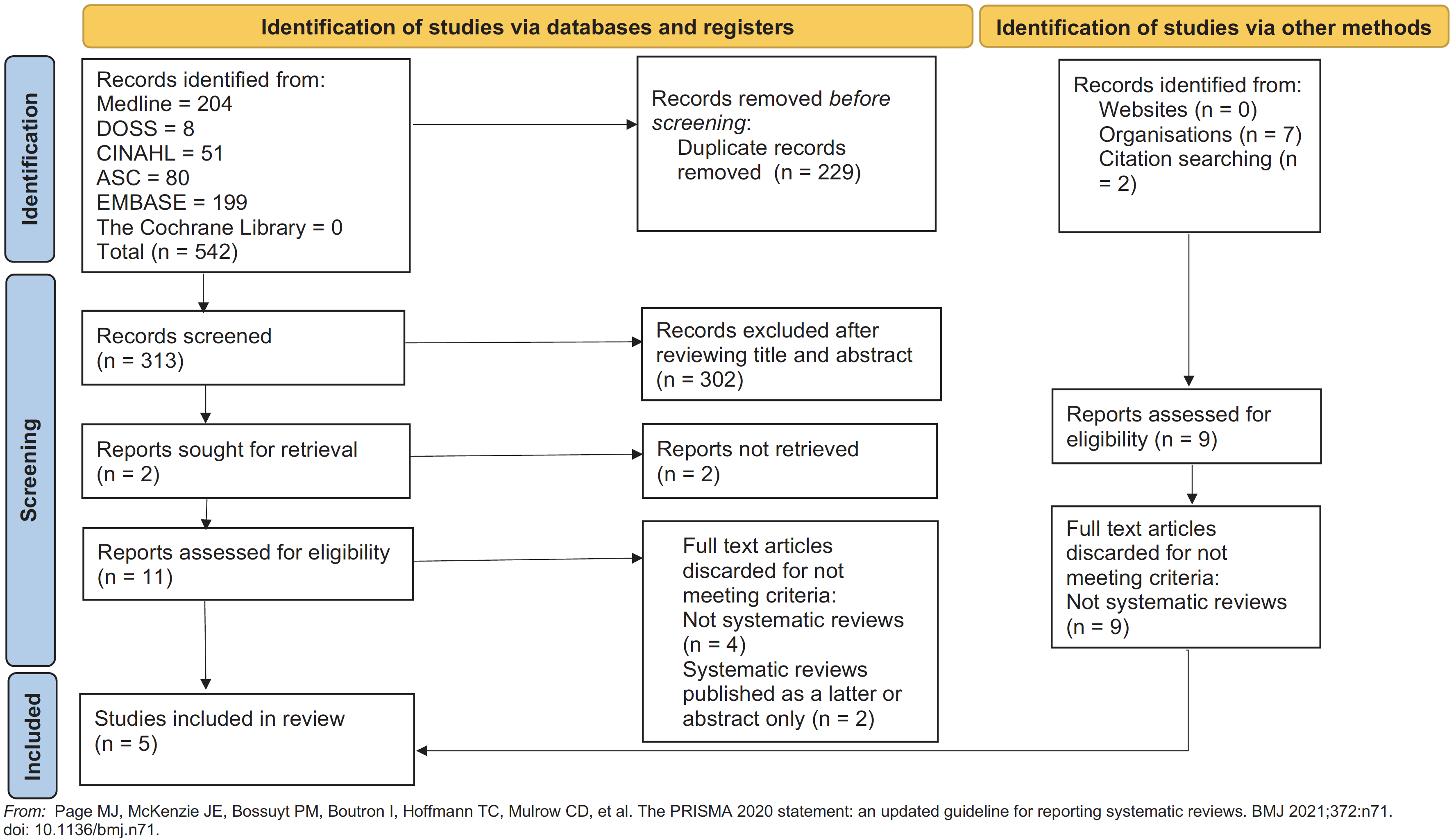 Fig. 1 PRISMA flowchart for selecting eligible studies
Fig. 1 PRISMA flowchart for selecting eligible studies
Systematic review selection
Five studies were ultimately included in this review after full text analysis, assessment of the inclusion and exclusion criteria and evaluation of their relevance to the research question. The process of study selection has been summarised in Figure 1.
Systematic review characteristics
All included studies were systematic reviews, with four including meta-analysis and one opting for narrative synthesis. Three of the five studies focused on comparing the use of remimazolam to midazolam alone, while two studies also compared remimazolam to propofol. These still included data directly comparing remimazolam to midazolam, which allowed for their inclusion. One systematic review was excluded in screening phase as it grouped midazolam with propofol into a combined ‘traditional sedatives’ group, which made direct comparison with midazolam impossible.9 Only one study was written from a dental perspective12 while four were written from a broader anaesthetics perspective, with a particular focus on the use of remimazolam in endoscopy, bronchoscopy or colonoscopy.13,14,15,16 These are the procedures for which procedural sedation is most commonly used outside of dentistry, and it is on sedation for these purposes that the majority of primary studies included in the reviews were reporting. All studies were reported in English between 2021 and 2022. A full summary of study characteristics is presented in Table 2.
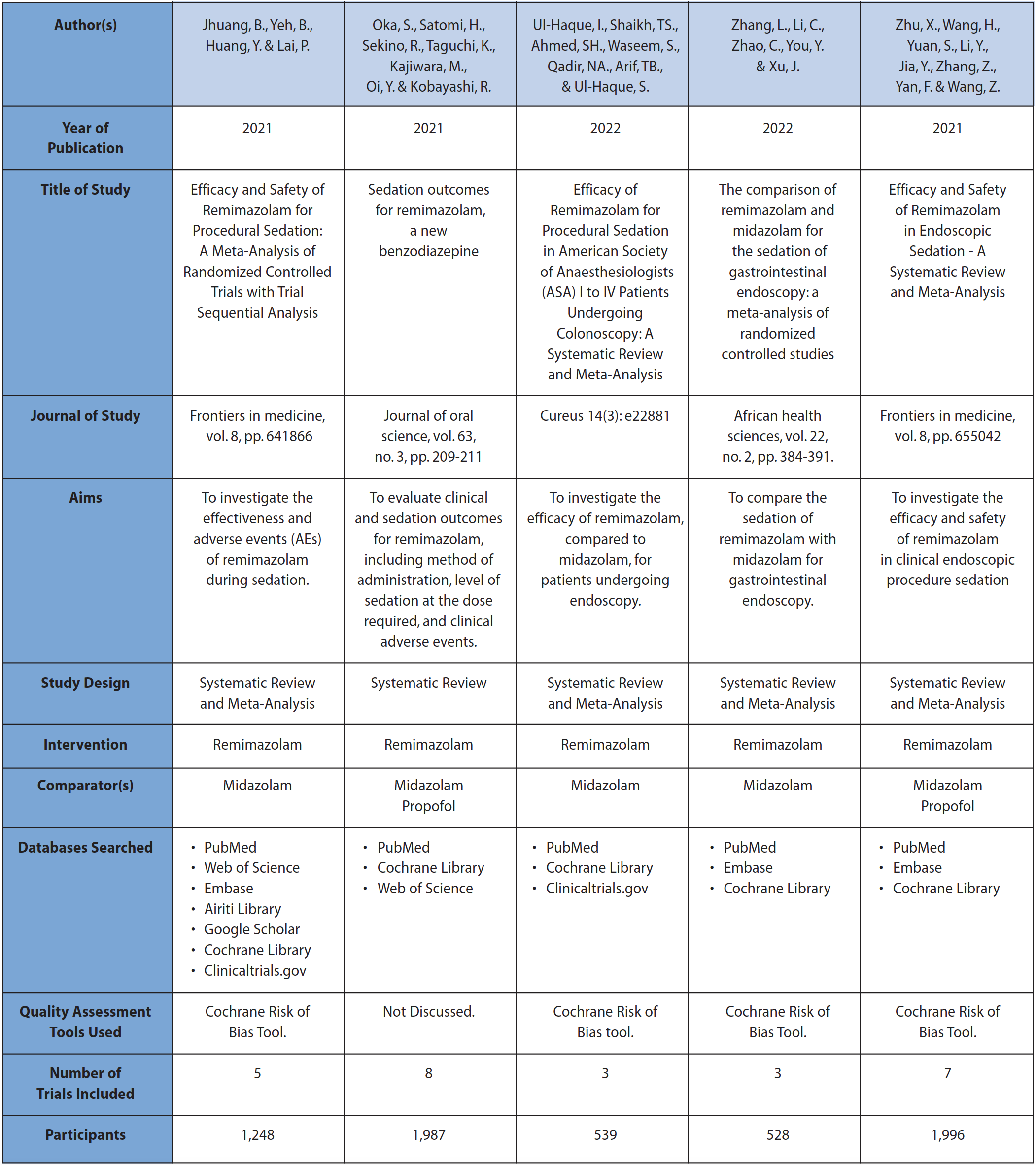 Table 2 Summary of study characteristics
Table 2 Summary of study characteristics
Risk of bias within systematic reviews
All systematic reviews were assessed for risk of bias using the ROBIS (risk of bias in systematic reviews) tool. Overall, three of the studies were assessed as having a low risk of bias, one study had a moderate risk of bias15 while one was considered to have a high risk of bias.12 The results from the risk of bias assessment are shown in Figure 2.
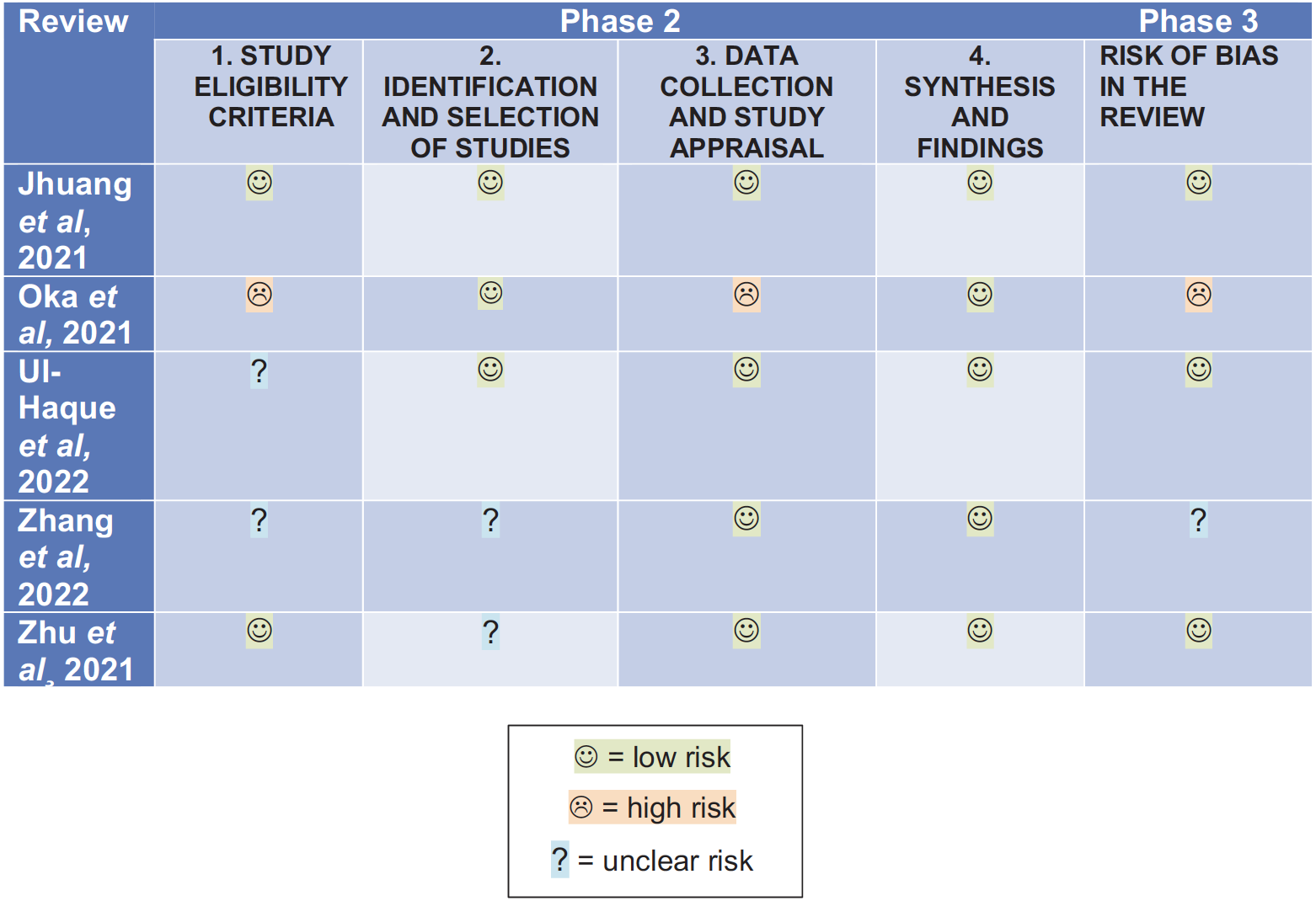 Fig. 2 Tabular presentation of ROBIS risk of bias assessment
Fig. 2 Tabular presentation of ROBIS risk of bias assessment
Characteristics of intervention
Remimazolam was administered intravenously in all included studies. Some studies used fentanyl as an adjuvant opioid analgesic.12,15 The chosen dose of remimazolam varied significantly across studies, ranging from 0.04 to 0.2 mg/kg given intravenously over one minute to a single intravenous bolus of 5 mg with a potential top-up of 2.5 to 3 mg.14,17,18 Dose-finding studies (clinical trials designed to determine the optimal or most effective dose of a medication) were conducted, using incremental groups based on weight or specific doses.17,18 One review only included dose-specific trials in their analysis, using an initial loading dose of 5 mg remimazolam with a top-up of 3 mg.14
Primary outcomes
Onset time
Among the mentioned systematic reviews, only one study12 provides a direct comparison of onset time between remimazolam and midazolam. According to this study, remimazolam achieves an optimal level of sedation more rapidly than midazolam, ranging from 1.5 to 6.4 minutes. Within this review, two primary studies directly compared the onset time, with remimazolam demonstrating faster onset compared to midazolam: 1.5 to 2 minutes versus 5 minutes17 and 2.2 to 2.6 minutes versus 4.8 minutes.18
Procedure success
Four reviews contained procedure success as an outcome measure. The definition of procedure success is elaborated in all studies except one13 and is a composite score measuring efficacy of the sedative. This score comprised between three and four outcomes. The first three of these four outcomes are consistent across the studies, with only one review14 omitting the fourth outcome:
1. Modified Observer’s Assessment of Alertness / Sedation (MOAA/S) ≤4 on 3 consecutive measurements taken every minute
2. Completion of the procedure
3. No requirement for an alternative and/or rescue medication
4. No manual or mechanical ventilation
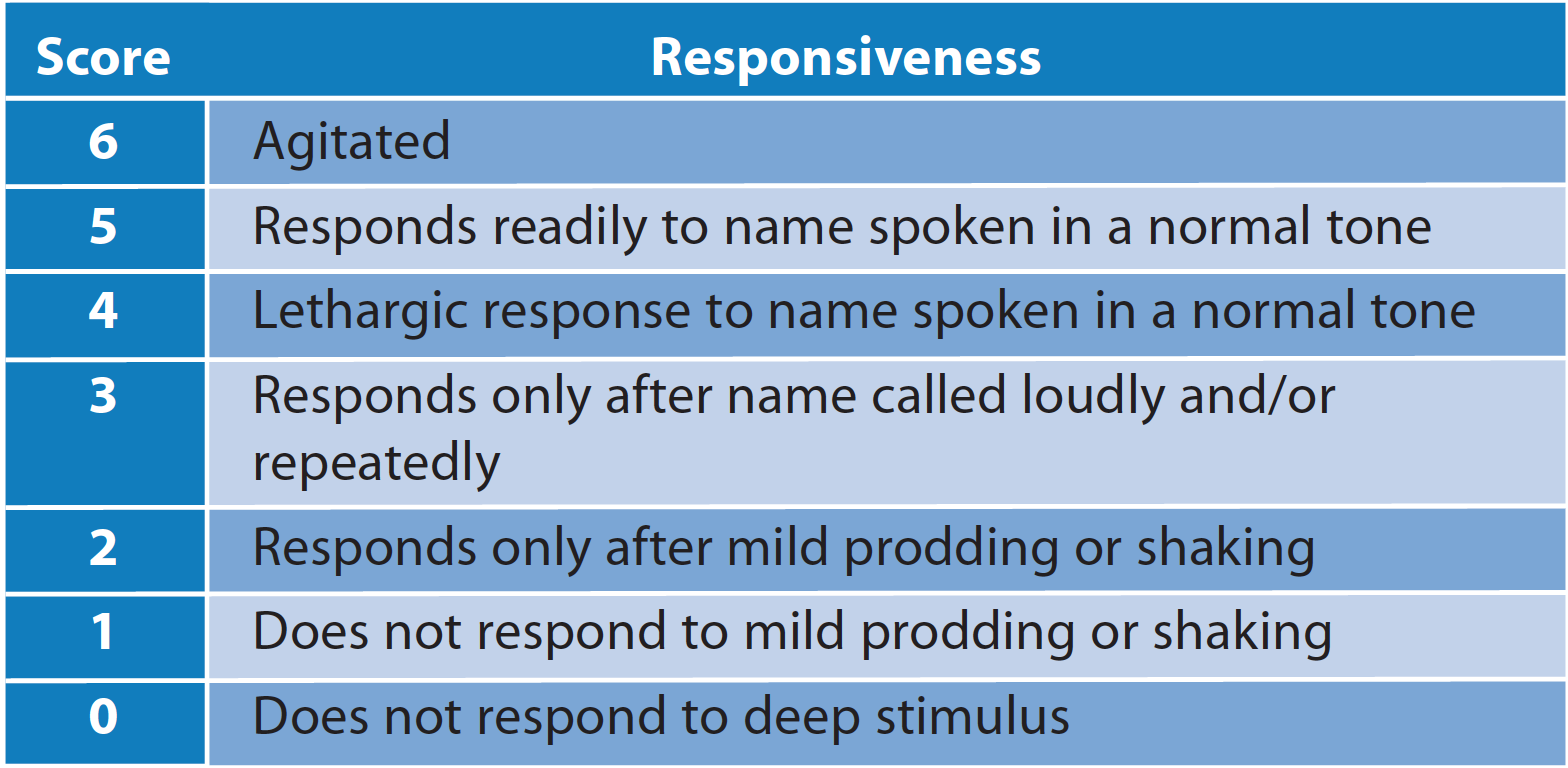 Fig. 3 The Modified Observer's Assessment of Alertness / Sedation (MOAA/S)
Fig. 3 The Modified Observer's Assessment of Alertness / Sedation (MOAA/S)
Two reviews13,16 found that remimazolam was superior to midazolam both before and after adjustment for heterogeneity (differences or variations in the data). One study14 initially found a statistically non significant result, but upon removing a dose-finding study with various doses of remimazolam compared to a fixed dose of midazolam18 from the analysis, sensitivity analysis (a test of the robustness or reliability of the study's results and conclusions) then strongly favoured remimazolam. The dose-finding study was removed from analysis as the authors stated that there is no advantage in dosing healthy individuals by weight rather than a specific dose.14 One review, which looked exclusively at remimazolam compared to midazolam for the purposes of endoscopy, found a statistically significant improvement in procedure success after sensitivity analysis with no remaining heterogeneity.15 It follows that all four included systematic reviews which measured procedure success found statistically significant superiority in favour of remimazolam when compared to midazolam.
Completion of procedure
Two systematic reviews13,14 included a distinct outcome measure for the completion of the procedure being carried out under sedation. Neither study, however, found a statistically significant association between remimazolam and the completion of procedures either before, or after, sensitivity analysis.
Dose required for adequate sedation
All studies defined adequate sedation as a Modified Observer’s Assessment of Alertness / Sedation (MOAA/S) score of 3 or ≤4. Only one study12 specifically assessed the dose required for adequate sedation as a specific outcome measure, and defined adequate sedation as a MOAA/S score of 3. It was stated that the minimum dose to achieve this was either 0.04 to 0.2 mg/kg given intravenously over one minute, or a 5 mg bolus given with or without a 2.5 mg top-up dose. Another review agreed that an initial dose of 5 mg bolus followed by up to 3 mg top-up had the highest efficacy when compared to other doses.15
Not exceeding assigned top-up dose
Only one review14 compared remimazolam to midazolam with regards to whether it was ever required to exceed the assigned top-up dose in order to achieve the desired level of sedation. A statistically significant result was found, with remimazolam comparing favourably to midazolam. This would suggest that it is less common for a prescribed dose of remimazolam to fail to achieve the level of sedation required in the patient without further top-ups.
Administration of rescue medication
Rescue medication is given if the initial dose of sedative given does not achieve adequate sedation for the completion of the surgical or investigative procedure. This is generally given as an unassigned top-up dose of the sedative in question.15 However, rescue medication may have been given as an alternative sedative; as such it was measured as a different outcome measure than assigned top-up dose. The use of rescue medication in patients receiving remimazolam or midazolam was compared in three of the included systematic reviews. One review found that the use of rescue medication was significantly reduced in the remimazolam group.13 Another carried out sensitivity analysis and found a significant reduction in the need for rescue sedatives both before and after this.14 One review quantified this reduced need for rescue sedatives as a reduction of 2.5 to 7.5%, resulting in a smoother sedation workflow compared to midazolam.15
Secondary outcomes
Time to recovery
Patients sedated with remimazolam tended to recover more quickly than those sedated with midazolam; one review13 found this difference to be statistically significant. Time to recovery from remimazolam sedation ranged from 6.8 minutes to 13.6 minutes; recovery from midazolam sedation ranged from 11.5 to 15.8 minutes.12 The dosage regimes across the primary studies were not consistent and so the result as to specific recovery times is not reliable.
Cognitive recovery
The recovery of cognitive function was assessed in two systematic reviews using the Hopkins Verbal Learning Test-Revised (HVLT-R). The HVL-T is a neuropsychological assessment tool used to evaluate verbal learning, memory and recognition abilities by presenting word lists and measuring immediate and delayed recall (around 20 to 25 minutes later) of the presented words as well as recognition accuracy.13,15 It was found that patients in the remimazolam group achieved significantly faster cognitive recovery than the midazolam group.13 Patients who were administered remimazolam demonstrated a shorter total recall and delayed recall, but it did not show any significant effect on attaining full alertness.15
Adverse events
No statistically significant difference was observed between remimazolam and midazolam regarding adverse events, both when considered as a whole or when analysing individual adverse event outcomes such as decreased oxygen saturation, headache, hypotension, hypertension, or bradycardia.13 However, one review noted a significantly lower risk of hypotension in the remimazolam group compared to midazolam, while no significant difference was found across other adverse event outcomes.16 Another review reported a lower risk of both hypotension and other adverse events in the remimazolam group15. Overall, remimazolam appeared at least non-inferior, and potentially safer, than midazolam.
Discussion
The pooled findings from five systematic reviews suggest that remimazolam may offer significant advantages over midazolam in terms of speed of onset, procedure success, rescue sedatives, recovery time, cognitive recovery and certain adverse events (hypoxia). However, it is important to note that only one systematic review considers remimazolam from a dental perspective, and no primary research included in the reviews takes place in a dental setting. Therefore, caution is needed when considering these results in the context of dental practice.
The choice of analgesic modality varies depending on the clinical context. While some primary studies used the opioid fentanyl for pain relief during sedation, in the dental setting, only local anaesthetics are typically used in combination with a benzodiazepine sedative. Opioids carry a higher risk of respiratory depression and hypotension,12 which may impact sedation depth and adverse events. Therefore, results regarding sedation depth, recovery and adverse events in studies in which fentanyl was used must be interpreted cautiously.
The primary research included in the systematic reviews focuses on remimazolam sedation for bronchoscopy, endoscopy, and colonoscopy, which are not analogous to dental procedures. Dental procedures often have longer durations and unique aspects such as fluids in the oral cavity which may influence adverse events associated with sedation. There is currently no clinical trial examining the use of remimazolam during dental procedures in primary care. While one randomised controlled trial in an outpatient oral and maxillofacial surgery unit showed increased procedure success rate and faster recovery with remimazolam, the difference in recovery time may not be clinically significant.8 Additionally, the oral surgery procedures in this trial represent only a fraction of those which may be performed in general dental practice. Therefore, further primary research focused on dental practice is needed.
The required dose for adequate sedation with remimazolam does not appear significantly different from that of midazolam. However, the mode of administration varies across the studies. Remimazolam is either given as a measured dose based on patient weight or as a fixed bolus with, or without, subsequent top-ups. The recommended standard for the dental setting is careful titration of the sedative to patient response, which has not been investigated in remimazolam studies. Further studies in this area are necessary.
While remimazolam has been associated with faster recovery time, there is no discussion of the time required for full psychomotor recovery, which is an important consideration. Reducing the recovery time may offer advantages to patients and their escorts in terms of supervision, work leave and transportation options.
The use of remimazolam for single-drug sedation is only discussed for adults in the IACSD 2023 statement. However, midazolam sedation is currently available for children over 12 years of age; research on the practicalities of sedating the 12 to 18-year-old age group with remimazolam would be beneficial.
Economic analysis is crucial to determine if the benefits of remimazolam are worth the financial cost. The economic arguments for remimazolam were not deemed sufficiently robust to approve its use within NHS Scotland for colonoscopy or bronchoscopy.11 However, this assessment does not consider its use as a dental sedative, and the economic analysis may not be generalisable to the dental setting.
Despite its drawbacks, midazolam has long been the drug of choice for procedural sedation in the dental setting due to its safety, predictability, affordability and familiarity to practitioners. To replace midazolam, remimazolam must demonstrate clear advantages while at least being non-inferior in other aspects. While remimazolam appears to have significant advantages, further primary research in the dental setting is necessary to determine if these advantages are clinically and practically significant enough for widespread adoption.
Limitations of this study include the inclusion only of English language research, which may have restricted access to valuable findings in other languages. Additionally, there were a small number of primary studies included across the systematic reviews, and there was overlap of primary studies across the reviews, potentially biasing the results. Publication bias must be considered due to the funding sources of the included primary research. Six out of eight primary studies across the reviews were funded by PAION UK Ltd., the manufacturer of remimazolam. Therefore, the conclusions of this review must be interpreted with caution.
Conclusion
The available evidence suggests that remimazolam has statistically significant advantages over midazolam for intravenous sedation. However, there is a lack of research on the use of remimazolam specifically in the dental setting and whether these advantages remain clinically significant. Further trials comparing remimazolam to midazolam in the dental setting, without concomitant opioids or sedatives and with titration of the drug, are needed.
Based on the discussed findings, future randomised controlled trials comparing remimazolam to midazolam for single-drug intravenous sedation in the dental setting are proposed. These trials should assess remimazolam's performance during longer dental procedures without concomitant systemic anaesthetics. It is important to evaluate whether remimazolam provides a meaningful advantage over midazolam; the most significant advantage may be the reduced time to recovery. Studies are needed to determine the time from induction to full cognitive and motor recovery following remimazolam sedation, in order to fully understand the potential benefits of this new medication.
Acknowledgements
This paper was derived from a dissertation written and submitted for consideration for the award of the degree of MSc in Oral Surgery from the University of Central Lancashire. The author would like to acknowledge his advisor, Professor Kathryn Taylor, for her help throughout this process.
Conflicts of Interest
The author has no conflicts of interest to declare.
References
1. Sneyd J R, Gambus P L, Rigby-Jones A E. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth 2021; 127: 41-55.
2. Illing K. Anxiety Management and Sedation in Dentistry; the next 60 years? SAAD Digest. 2018; 34: 47-50.
3. Dao V A. Efficacy of remimazolam versus midazolam for procedural sedation: post hoc integrated analyses of three phase 3 clinical trials. Endosc Int Open. 2021; 10: E378-E385.
4. Lee A, Shirley M. Remimazolam: A Review in Procedural Sedation. Drugs. 2021; 81: 1193-1201.
5. Kim S H, Fechner J. Remimazolam – current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J Anesthesiol. 2023; 75: 307-315.
6. Intercollegiate Advisory Committee for Sedation in Dentistry. Standards for Conscious Sedation in the Provision of Dental Care. 2020. Available from: https://www.saad.org.uk/IACSD%202020.pdf [Accessed 10/04/2023].
7. Intercollegiate Advisory Committee for Sedation in Dentistry. Remimazolam for intravenous conscious sedation for dental procedures. 2023. Available from: https://www.rcseng.ac.uk/-/media/fds/iacsd/iacsd-remimazolam-statement-130623.pdf [Accessed 18/10/2023].
8. Guo Z, Wang X, Wang L, Liu Y, Yang X. Can Remimazolam Be a New Sedative Option for Outpatients Undergoing Ambulatory Oral and Maxillofacial Surgery? J Oral Maxillofac Surg. 2022; 81: 8-16.
9. Tang Y, Yang X, Yu Y, Shu H, Xu J, Li R, Zou X, Yuan S, Shang Y. Remimazolam versus traditional sedatives for procedural sedation: a systematic review and meta-analysis of efficacy and safety outcomes. Minerva Anestesiol. 2022; 88: 939-949.
10. Paion. Paion launches Byfavo® (Remimazolam) in the UK for procedural sedation. 2021. Available from: https://www.paion.com/newsdetails/paion-launches-byfavo-r-remimazolam-in-the-uk-for-procedural-sedation/?cHash=04b6c8 adc0779e2e5dc273936a459a6d [Accessed 27 November 2022].
11. Scottish Medicines Consortium. Remimazolam 20mg powder for solution for injection (Byfavo®). Healthcare Improvement Scotland. 2022. Available from: https://www.scottishmedicines.org.uk/media/7040/remimazolam-byfavo-final-july-2022-for-website.pdf.
12. Oka S, Satomi H, Sekino R, Taguchi K, Kajiwara M, Oi Y, Kobayashi R. Sedation outcomes for remimazolam, a new benzodiazepine. J Oral Sci. 2021; 63: 209-211.
13. Jhuang B, Yeh B, Huang Y, Lai P. Efficacy and Safety of Remimazolam for Procedural Sedation: A Meta-Analysis of Randomized Controlled Trials With Trial Sequential Analysis. Front Med. 2021; 8: 641866.
14. Ul-Haque I, Shaikh T G, Ahmed S H, Waseem S, Qadir N A, Bin Arif T, Haque S U. Efficacy of Remimazolam for Procedural Sedation in American Society of Anesthesiologists (ASA) I to IV Patients Undergoing Colonoscopy: A Systematic Review and Meta-Analysis. Cureus. 2022; 14: e22881.
15. Zhang L, Li C, Zhao C, You Y, Xu J. The comparison of remimazolam and midazolam for the sedation of gastrointestinal endoscopy: a meta-analysis of randomized controlled studies. Afr Health Sci. 2022; 22: 384-391.
16. Zhu X, Wang H, Yuan S, Li Y, Jia Y, Zhang Z, Yan F, Wang Z. Efficacy and Safety of Remimazolam in Endoscopic Sedation—A Systematic Review and Meta-Analysis. Front Med. 2021: 65504.
17. Borkett K M, Riff D S, Schwartz H I, Winkle P J, Pambianco D J, Lees J P, Wilhelm-Ogunbiyi K. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015; 120: 771-780.
18. Pambianco D J, Borkett K M, Riff D S, Winkle P J, Schwartz H I, Melson T I, Wilhelm-Ogunbiyi K. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016; 83: 984-99.