Please click on the tables and figures to enlarge
Dexmedetomidine: pharmacology and use as a sedative agent
Charlotte Richards BDS, MFDS RCPSG, Dip Con Sed, FHEA*
Clinical Lecturer and Specialty Registrar Oral Surgery, Cardiff University, School of Dentistry, Heath Park, Cardiff, CF14 4XY
*Correspondence to: Charlotte Richards
Email: richardsc34@cardiff.ac.uk
Richards C. Dexmedetomidine: pharmacology and use as a sedative agent. SAAD Dig. 2024: 40(1): 69-76
Abstract
With the recent introduction of remimazolam into conscious sedation for dentistry there is a natural interest in the possibility of other drugs suitable for sedation in the field. This review explores the drug dexmedetomidine, an alpha adrenoceptor agonist first approved for use in intensive care units for short term sedation on intubated patients. It has sedative, analgesic, anxiolytic and antihypertensive properties. Interest was first sparked in dexmedetomidine as it was merited for having minimal impact on respiration, in comparison to midazolam which causes respiratory depression.
This review explores the literature of the pharmacodynamic and pharmacokinetic properties, followed by evaluation of the five available randomised control trials in the literature that compare midazolam with dexmedetomidine for procedural sedation in dentistry.
Overall, although dexmedetomidine seems to be associated with improved patient satisfaction and operator satisfaction with patients more relaxed during treatment, the limitations of being unable to use this drug as an operator sedation technique and the potential significant cardiovascular changes mean that dexmedetomidine is unlikely to ever become a drug of choice for intravenous conscious sedation in dentistry.
Introduction
Dexmedetomidine (Precedex®) is an alpha (∝) adrenoceptor agonist, which is highly selective for ∝2 receptors, with an ∝2:∝1 selectivity ratio of 1620:1. Alpha 2 adrenoceptors have sympatholytic properties, and the receptors are present both centrally and peripherally. Activation of ∝2 receptors inhibits adenylyl cyclase, ultimately suppressing ascending noradrenaline pathways. Through this action it has sedative, analgesic, anxiolytic and antihypertensive effects. The drug was first approved for use in intensive care units (ICU) for short term sedation on intubated patients by the Food and Drug Administration (FDA) in the United States in 1999, but since 2008 has been licenced for procedural sedation.
Pharmacokinetics
Dexmedetomidine is only licensed for intravenous use. It is highly soluble in water and requires dilution in water for injection by the sedationist. If taken orally, it undergoes extensive first pass metabolism with a subsequent 16% drug bioavailability.1 It crosses mucous membranes well, and there has been development of multiple off-licence applications with intranasal sprays via a mucosal atomiser device, buccal gel preparations and sublingual sprays. The bioavailability following administration onto the oral mucosa and nasal membranes has been shown to be 83% and 65% respectively.1
Pharmacokinetic studies have demonstrated wide variability between patients (Table 1). When administered intravenously it has a rapid initial distribution half-life (∝-half-life) of six minutes.1 The speed of onset is approximately 5 minutes, with peak effects at 15 minutes. 94% of the drug binds to plasma proteins such as albumin, comparable to 97% protein binding of midazolam. The drug is highly lipophilic and as such has a high volume of distribution of approximately 118 litres (L). This has been shown to be highly variable between patients particularly those in the ICU, from 79.3 to 389 L.
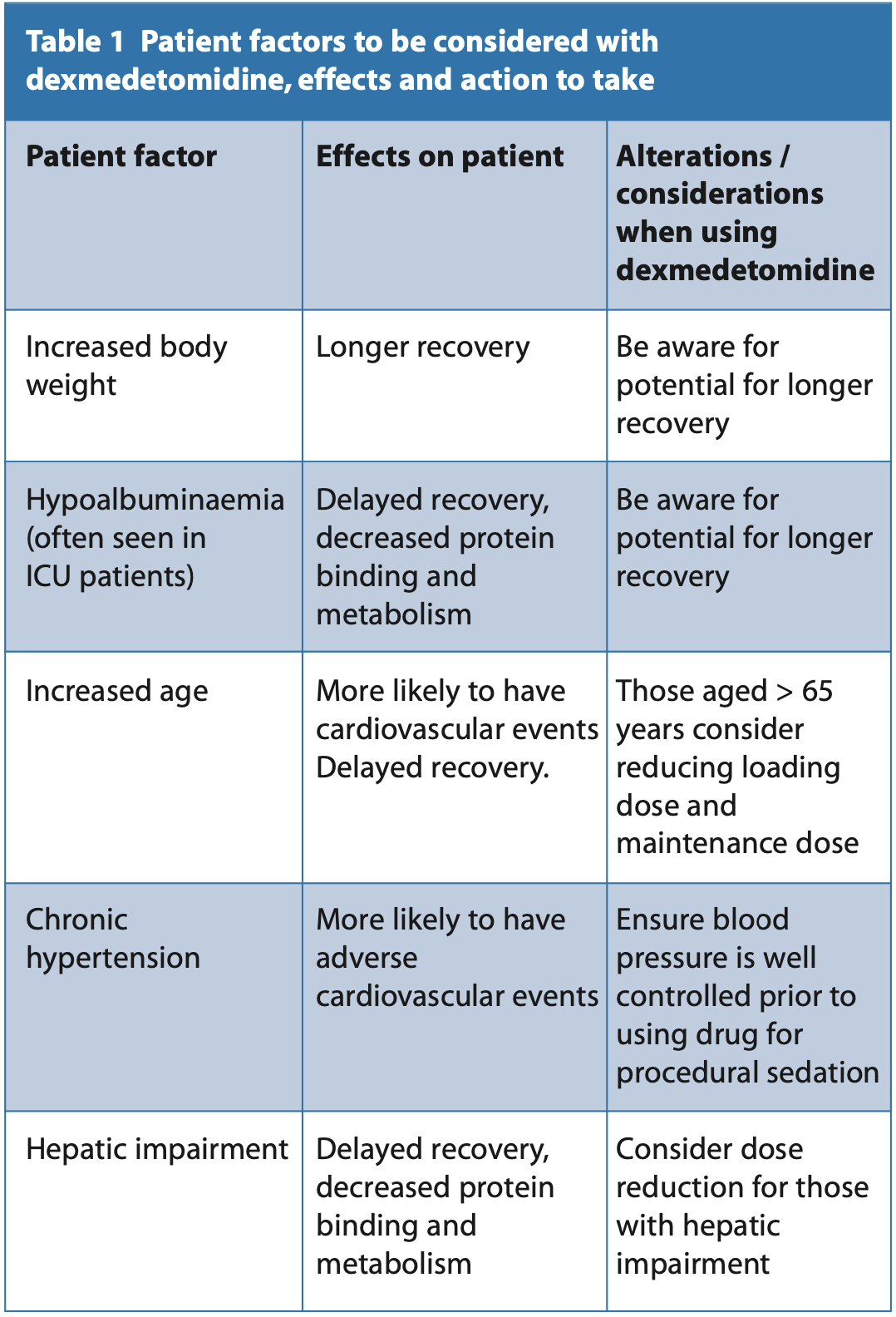
It is metabolised in the liver via glucuronidation and hydroxylation via the P450 cytochrome family of enzymes. It has an average elimination half-life (β-half-life) of two to two and a half hours. It does not produce any active metabolites and is mainly excreted via the kidneys, with 90% of the initial dose excreted as non-active metabolites in urine. The remaining 10% is excreted in the faeces. Recovery can vary depending on the length of infusion and there is conflicting evidence in the literature in terms of recovery. A randomised control trial comparing midazolam to dexmedetomidine for third molar surgery in the dental setting found that all patients were clinically fit for discharge within 30 minutes, comparable to midazolam.3 However, there had been reports of delayed recovery in studies in the dental setting with dexmedetomidine. One study providing oral surgery procedures for patients reports an average recovery of 82.2 minutes, and the shortest recovery of 57.9 minutes with a procedure length of 45 minutes. Variable recovery was also observed with a standard deviation of 24.3 minutes.4 It appears that there is high individual variability in recovery of patients due to variable elimination half- lives. This may be explained by the factors in Table 1.
Pharmacodynamics
Sedation and anxiolysis
∝2 receptors are located throughout the body, therefore the effects of dexmedetomidine are wide ranging. Dexmedetomidine has sedative effects by activation of pre- and post-synaptic ∝2 in the locus coeruleus in the brain. This nucleus in the brainstem is involved in the sleep cycle and is completely inactive in rapid eye movement sleep. This mechanism is thought to make sedation with dexmedetomidine close to natural sleep, but patients are still easily rousable with verbal stimuli.5 Multiple studies have recorded similar bispectral index scores (BIS) which monitor the depth of sedation recorded at between approximately 80 to 85 at optimal sedation for procedural sedation.3,6 This is consistent with values expected for light to moderate sedation.7 Sedative effects are dose dependent, with rousable sedation associated with a plasma concentration of approximately 0.2 to 0.3 ng/ml and deep, unrousable sedation at plasma concentrations above 1.9 ng ml.5 Dexmedetomidine has anxiolytic properties, which will be discussed in the context of comparison to midazolam in the dental setting later.
Analgesia
∝2 receptors are activated centrally and in the spinal cord. Activation suppresses pain transmission both via reduction of action potential firing along peripheral A delta and C fibres, and inhibition of pre-synaptic receptors in the spinal cord, thus reducing pain signals to the brain. Multiple studies have demonstrated the analgesic properties of dexmedetomidine, and analgesic applications have been used in the pre-operative, intra- operative and post-operative period. In the ICU setting, dexmedetomidine is useful to reduce the overall dose of opioids that patients require, reducing recovery time.8 Healthy volunteers in a randomised control trial had 30% less pain to a cold test than placebo drug.9 Patients having third molar surgery with pre-operative intranasal dexmedetomidine had significantly reduced postoperative pain compared to the local anaesthetic alone group.10
Effects on the cardiovascular system
Through inhibition of the sympathetic nervous system, the drug has effects on the cardiovascular system, which can result in bradycardias and hypotension. ∝2 adrenergic agonists have an interesting biphasic haemodynamic effect on the cardiovascular system with initial hypertension, followed by return to baseline and then hypotension.
A study of healthy volunteers given increasing doses of dexmedetomidine showed a dose-dependent relationship with blood pressure. Following the initial IV bolus hypertension was observed with the highest two doses (1.0 μg/kg, 2.0 μg/kg), with a return to baseline on average seven minutes post the start of the sedation, followed by hypotension. All doses resulted in a reduction in blood pressure, which was at its maximum 60 minutes post sedation commencement. The highest dose at 2.0 μg/kg was associated with the largest subsequent blood pressure fall. Overall, mean arterial blood pressure was significantly reduced in all dose groups.11 This biphasic response is thought to be due to initial vasoconstriction in the peripheral vasculature, followed by initiation of central and peripheral ∝2 receptors inhibiting the sympathetic nervous system, resulting in reduced systemic vasculature resistance. The study found reduced plasma concentrations of adrenaline and noradrenaline which may explain the prolonged hypotension seen in the participants.
In the same study, as blood pressure increases at the start of in the infusion, a dose-dependent fall in heart rate also occurred, with the largest fall from 59 beats per minute (bpm), to 43 bpm in the 2.0 μg/kg dose group, presumably due to activation of the baroreceptor reflex. Heart rate increases after the initial dip, as blood pressure decreases, but participants remain bradycardic throughout infusion. Likewise cardiac output was significantly reduced during the initial infusion. In addition, there were four electrocardiogram (ECG) changes in the highest dose group, although none required any clinical treatment.
Given the cardiac changes seen with infusion of dexmedetomidine, the British National Formulary recommends monitoring of cardiac function.12 Although this is not specific in terms of the monitoring required, ECG monitoring and pre-, intra- and post-operative blood pressure measures would be sensible. Dexmedetomidine is contraindicated in patients with pre-existing bradycardia such as those with a beta blocker induced bradycardia and patients with known hypotension.
Effects on airway and respiratory depression
As the major disadvantage of midazolam is that of respiratory depression, an agent that produces comparable sedation, but less respiratory depression would be an excellent alternative.
Initially dexmedetomidine was merited for having minimal impact on respiration and the risk of airway collapse. Much of this initial data was based on a double-blinded randomised control trial in 1992 with 37 healthy males who were administered intravenous infusions of dexmedetomidine, at increasing doses.13 Ventilation was monitored alongside inspired and expired oxygen and carbon dioxide concentrations throughout. The study concluded that no patients significantly desaturated (<90% SpO2), there was only mild hypercarbia and only mild decreases in ventilation. However, this was dose-dependent. At the two highest doses, seven out of ten subjects (2.0 μg/kg) and five out of six subjects (1.0 μg/kg) had transient obstructive apnoeas immediately after infusion. Dose- dependent effects on tidal volume were demonstrated, with a large decrease in tidal volume immediately following the initial infusion. In addition, at the two higher doses 67% of patients fell asleep. This was determined if patients were unrousable by normal voice command and, as such, the ability to ask patients to take deep breaths to simply overcome the transient apnoeas may be more troublesome. It should be noted that a limitation of this study is that the subjects were all fit and healthy, with no underlying respiratory conditions and the results cannot be extrapolated to the general population.
A 2019 non-blinded randomised control trial crossover study with nine healthy volunteers assessed airway collapsibility and respiratory depression, comparing dexmedetomidine and propofol infusions.14 All participants were given infusions of dexmedetomidine to induce sedation at a BIS mean of 74 for light sedation and 57 for moderate sedation. Oxygen saturations did not decrease below 96% at any time during any infusion. The data of interest for the application of procedural sedation is that of lower infusions inducing a light sedation. This study used a methodology to assess upper airway collapsibility, by assessing the pharyngeal pressure required to keep it open or closed.15 There was a large variation in pharyngeal critical pressures. A value above zero cm indicates upper airway obstruction at atmospheric pressure. For the low infusion with dexmedetomidine this varied from -15 cm to 2.3 cm, with an average of -2.0 cm, compared to -15 cm to 1.5 cm with an average of 0.9 cm with propofol. Three participants in the dexmedetomidine group had central apnoeic episodes during the initial bolus infusion, as in the 1992 study. However, it is difficult to make definitive conclusions with small sample sizes in these non-clinical settings.
There have been studies using dexmedetomidine for procedural sedation in dentistry and analysis of the effects on the airway and respiratory drive. A study comparing midazolam and dexmedetomidine for implant surgery observed a lower average mean Sp02 in the midazolam group but no significant difference between the two groups.16 A similar study in day case maxillofacial surgery found comparable Sp02 between midazolam and dexmedetomidine groups throughout induction, surgery and recovery.6
Amnesia
Dexmedetomidine has been associated with less post-operative delirium, as well as little amnesia compared to midazolam.16 In addition, in the ICU setting, patients have fewer problems with memory and improved cognitive function, compared to propofol.8
Monitoring, precautions and contraindications
Due to the cardiovascular changes during infusion, the Precedex® safety data sheet recommends continuous ECG, blood pressure and oxygen saturation monitoring throughout treatment.17 The drug is contraindicated in patients with pre-existing bradycardias, known hypotension, hypovolaemic patients and those with advanced heart block. One of the most serious adverse effects with dexmedetomidine is cardiac arrest, due to progression of the bradycardia to pulseless electrical activity and there are numerous case reports in the literature. These are mostly associated with patients over the age of 50 with known heart block or left ventricular dysfunction.18,19,20
Caution should also be exercised in those with impaired hepaticfunction, impaired renal function and those aged over 65, with reduction of the loading and maintenance dose.
Radioactive labelling studies show that dexmedetomidine crosses the placenta, but as with most drugs there is no evidence on the effects on the unborn foetus, and the manufacturer recommends avoiding unless there is no suitable alternative.
Dexmedetomidine can cause corneal dryness, nausea, and vomiting.
Summary of pharmacodynamic and pharmacokinetic properties
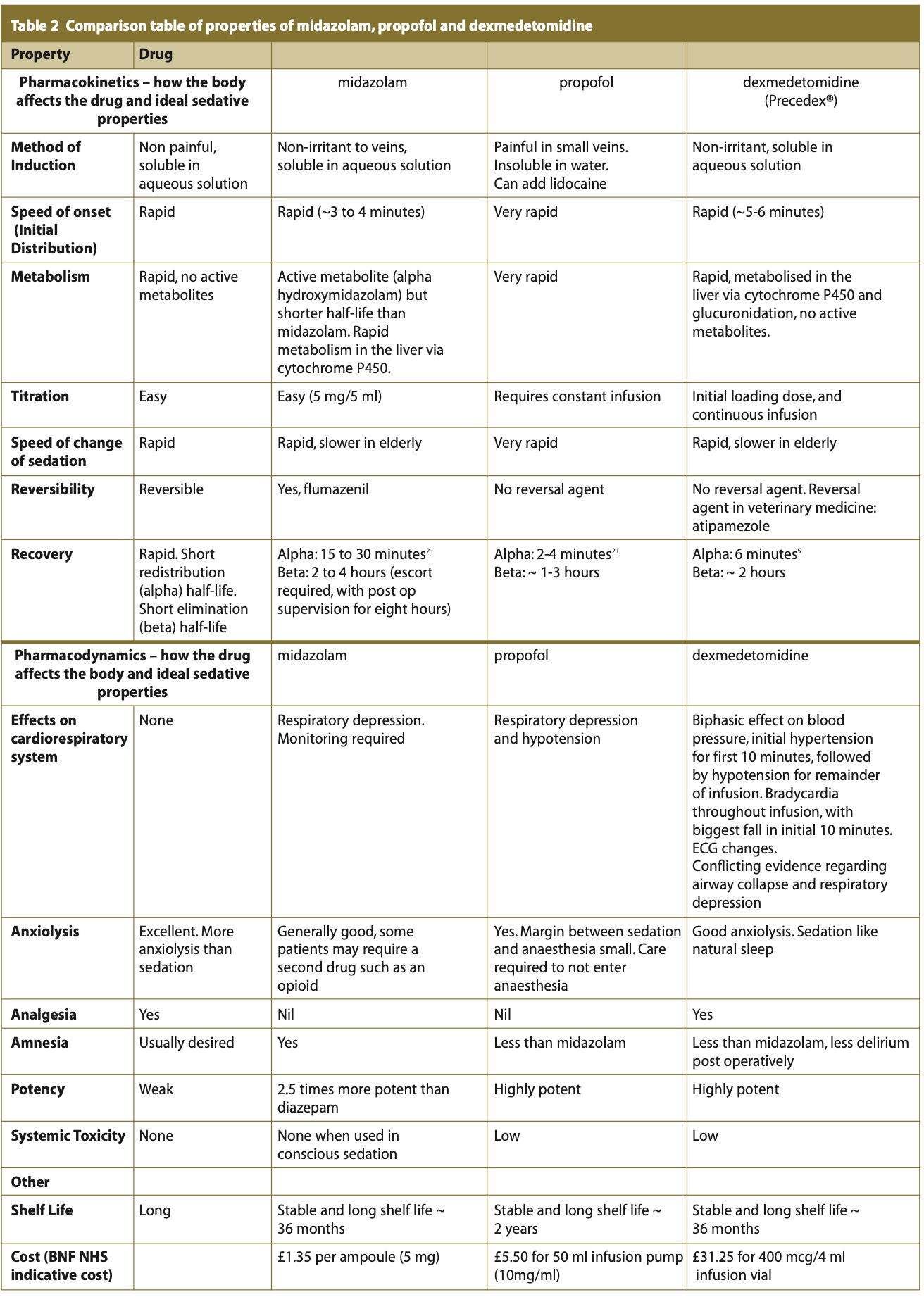
Table 2 summarises the pharmacodynamic and pharmacokinetic properties, compared to the ideal intravenous sedation agent alongside those of midazolam and propofol.
Use in the intensive care unit (ICU) and medicine
The first licenced application of dexmedetomidine was approved in 1999 by the FDA for short term sedation (<24 hours) and analgesia for mechanically ventilated patients in the ICU. Two large scale randomised, double-blinded clinical trials were set up across Europe from 2007 to 2010 to evaluate dexmedetomidine versus midazolam (MIDEX) and dexmedetomidine versus propofol (PRODEX) for light to moderate sedation in the intensive care setting. The results showed that dexmedetomidine was not inferior to either midazolam or propofol.
In the MIDEX study the duration of mechanical ventilation was significantly shorter with dexmedetomidine compared to midazolam, and although shorter in the PRODEX study this was not statistically significant. The median ICU length stay was shorter for dexmedetomidine, but not statistically significant. Nurses evaluated patient co-operation, pain communication and arousal to be better with dexmedetomidine. In addition, post extubation neurocognition was evaluated.
In the PRODEX trial patients received treatment for agitation, anxiety and delirium less frequently with dexmedetomidine compared to propofol. There was no difference in the MIDEX trial.22,23 Although cost effectiveness analysis did not form part of the trial it could be postulated that dexmedetomidine may be more cost effective in comparison to midazolam with a shorter ICU stay, and in comparison to propofol with less treatment required for post extubation anxiety and delirium.
The FDA expanded the licence for dexmedetomidine to non- intubated patients requiring procedural sedation in 2008. There has been use of dexmedetomidine across multiple medical and surgical specialities including the Emergency Department, endoscopy, paediatrics, ophthalmic surgery, urology, anaesthetics (for awake fibre optic intubation), radiology and general surgery.
Application in endoscopy may be particularly useful, where traditionally midazolam and an opioid such as fentanyl are used. These drugs when combined can result in significant respiratory depression and resulted in a higher number of deaths than expected in the 2004 report of the National Confidential Enquiry into Patient Outcome and Death, making recommendations to the practice of sedation in endoscopy.24 Given that dexmedetomidine has analgesic properties with opioid sparing effects, it may be a highly suitable alternative for this patient group. A randomised control trial comparing midazolam with dexmedetomidine for upper gastrointestinal endoscopy found significantly higher mean oxygen saturations, better patient satisfaction and higher quality sedation.25
A recent licence has been added in 2022 for acute agitation in bipolar and schizophrenic disorders by sublingual preparation (Igalmi®), following excellent results in double-blinded randomisedcontrol trials.26
Use in dentistry
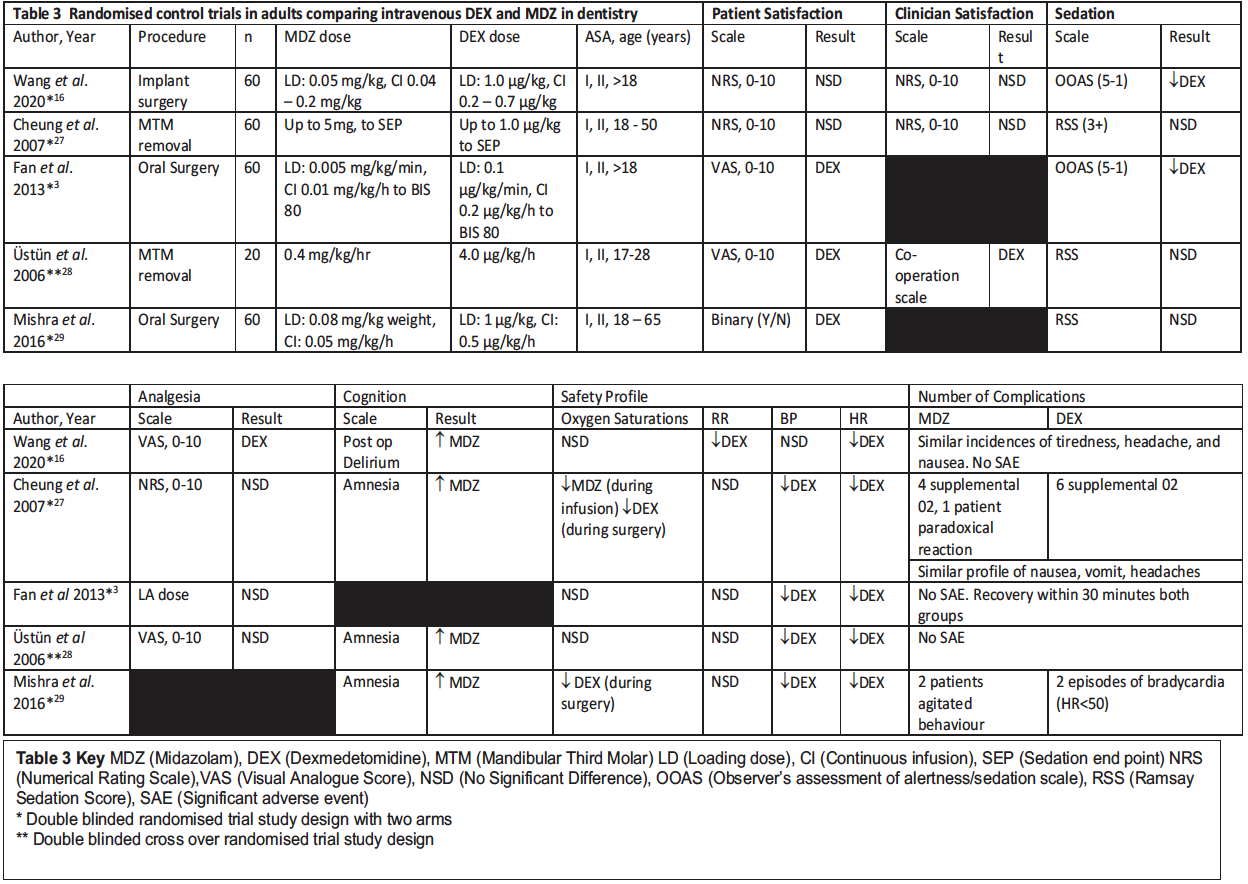
Midazolam has been the unrivalled intravenous single drug of choice for conscious sedation in dentistry, therefore dexmedetomidine has been compared to midazolam in randomised control trials in dentistry for conscious sedation. Following a literature search, Table 3 displays and compares the results of the five studies available in the literature comparing intravenous midazolam to intravenous dexmedetomidine in adult populations for dental procedures under conscious sedation.
When interpreting the results in Table 3 it should be noted that all studies had similar, strict inclusion criteria with all patients allocated as American Society of Anesthesiologists (ASA) physical classification status I or II, fit and healthy, with no severe hepatic or renal impairment, not pregnant, no pre-existing sleep apnoea, no asthma, no diabetes mellitus and no clinical history or ECG evidence of heart block.
Patient and clinician satisfaction
In three out of five studies, patients significantly preferred sedation with dexmedetomidine. This was evaluated post-operatively, with the method of evaluation presented in Table 3. Consistently patients felt more relaxed and less anxious in the post-operative recovery period after dexmedetomidine. Three out of five studies assessed clinician satisfaction, with one study finding a significant result with clinicians preferring dexmedetomidine for their operating conditions.28 Other studies had no significant difference.
In two out of the five studies a lower score was achieved on the observer’s assessment of alertness / sedation scale (OOAS) indicating deeper sedation with dexmedetomidine. In one study, the OOAS was significantly lower between 2.0 and 3.0 for dexmedetomidine, compared to a 3.0 average score for midazolam.16 Similar results were found in the Fan, Ti and Islam3 study with a minimum OOAS of 2.53, and a corresponding BIS of 69.9 in the dexmedetomidine group compared to a minimums OOAs of 3.1 and a corresponding BIS of 76.4 in the midazolam group. Providing the patient can maintain verbal contact and consciousness during the sedation, dexmedetomidine may have advantages particularly with more unpleasant procedures if patients are more relaxed.
The other three studies found no significant difference in the assessment of sedation, although in one of these studies the maximum dose of midazolam given was 5 mg,27 and this is unlikely to be a high enough dose of midazolam for all patients for good sedation given that the average dose of midazolam in conscious sedation is 7 mg. The Ramsay Sedation Score (RSS) used in three papers for assessment of sedation was criticised in other papers due to the difficulty in being able to use this scale with patients undergoing oral surgery procedures and is more suited to the intensive care setting.
In the three studies that assessed amnesia, midazolam was associated with significantly more amnesia post-operatively. Although amnesia may be a desired characteristic for some unpleasant procedures, for patients with dental anxiety the amnesia can be counter-productive when they have coped well with treatment and being able to recall some of the positives would aid them with their anxiety. In addition, some patients find the memory loss unpleasant. Dexmedetomidine appears to be able to help with this patient group. Patients experienced less post-operative delirium in the Wang, Zhou, Zhang, Huang and Peng16 study which is also seen in the intensive care setting, and this may be contributing to the increased patient satisfaction post- operatively where patients seem more relaxed and less agitated.
Four studies reviewed analgesia, with only one study finding a significant result with reduced post-operative pain in the first few hours with dexmedetomidine, but this was unchanged on the day post surgery.16
Safety profile
Consistent changes in blood pressure and heart rate were seen across all five studies with significant decreases in mean arterial pressure and heart rate for dexmedetomidine groups, with two episodes of bradycardia in Mishra, Birmiwal, Pani, Raut, Sharma and Rath.29 Dexmedetomidine did not show any advantages over midazolam in terms of oxygen saturation or respiratory depression, as is consistent with more recent studies on the effects of dexmedetomidine on respiratory depression.15 In one study, six patients required supplemental oxygen with dexmedetomidine in comparison to four patients requiring supplemental oxygen with midazolam.27 Across all studies there were no significant adverse events which is perhaps not surprising given the cohort of patients included in the studies. Across all trials three patients experienced paradoxical reactions with midazolam which is not seen with dexmedetomidine.
Dexmedetomidine administration varies in the literature and clinical guidelines, but all suggested administrations in the context of procedural sedation for dentistry recommend an initial bolus infusion over approximately ten minutes, followed by a maintenance infusion, with some of the maintenance infusions adjusted to the patient response. All doses are weight dependent, with examples of infusions used in trials given below:
- Initial 1.0 µg/kg over a ten-minute period, surgery commencing, followed by maintenance infusion at 0.5 µg/kg per hour, with the infusion stopping at the time of suturing commencing.30
- Initial 1.0 µg/kg of dexmedetomidine over a ten-minute period, followed by a maintenance infusion at 0.2 - 0.7 µg/kg per hour until the end of surgery.16 The paper does not specify how the maintenance dose was chosen.
- Initial infusion of 0.1 µg/kg per minute until adequate sedation was achieved (determined by the operator), followed by a maintenance infusion of 0.2 µg/kg per hour.3
Having reviewed the studies, it consistently appears that the faster the initial bolus dose is given, the greater the dose-dependent cardiovascular effects.
Dexmedetomidine is specifically mentioned in both the United States and South African dental / procedural sedation guidelines. There is no specific guidance regarding its use in the United States, but it is recognised as an available drug to use for dental procedural sedation. In South Africa use of the drug is categorised as an advanced sedation technique, in comparison to midazolam which is categorised as a basic technique. In addition, the drug should only be used by those with ‘formal training in anaesthesia or intensive care medicine, or by experienced sedation practitioners with anaesthetic experience.’ The recommended dosing regimen in the South African guidance is 1 µg/kg over a ten-minute period, decreased to 0.5 µg/kg over a ten-minute period for those aged over 65 years followed by a maintenance infusion of 0.6 µg/kg per hour titrated to a clinical effect with a normal range of 0.2 µg/kg per hour. The guidance also recognises that dexmedetomidine is being used increasingly for nasal and buccal administration in children.31
Paediatric dentistry
Paediatric patients present different issues to adult patients in relation to sedation. They are highly likely to be needle phobic, unco-operative and can deteriorate quickly compared to adult patients. Although dexmedetomidine undergoes extensive first pass metabolism and cannot be used successfully orally, it crosses mucous membranes well and intranasal preparations can be very useful in paediatrics.
In paediatric general anaesthesia day cases for dental extractions dexmedetomidine used intranasally is the premedication of choice at Guys’ and St Thomas’ NHS Foundation Trust. Anecdotally it is associated with a reduced recovery time post general anaesthetic compared to midazolam used as a premedication. A study comparing premedication with oral midazolam to premedication with oral dexmedetomidine evaluated the Ramsay sedation scale; parental separation anxiety scale; mask acceptance scale and the paediatric anaesthesia emergence delirium scale. Both drugs were comparable in terms of sedation and anxiolysis. There was a significant decrease in heart rate in the dexmedetomidine group from baseline for the duration of the operation.32 In addition, there was a significant decrease in post anaesthesia emergence delirium with dexmedetomidine, which on a busy dental paediatric list could aid recovery turnover if children are more settled. Similar results were seen in a similar paediatric dentistry trial comparing intranasal dexmedetomidine with oral midazolam with a significant reduction in post general anaesthesia emergence delirium.33
Conclusions - how useful for dentistry in the UK?
Although now a generic drug, a prohibitive factor is likely to be cost with dexmedetomidine being 23 times the cost of midazolam and 6 times the cost of propofol. Cost-effectiveness would need to be proven. In dentistry, intravenous dexmedetomidine seems to be associated with improved patient satisfaction and patients that are more relaxed during treatment. Its analgesic properties could potentially be useful, although this was not seen in the randomised control trials. The lack of amnesia and more relaxed state of patients post procedure would be useful for some patient cohorts. In addition, paradoxical reactions are not seen as they are with midazolam and this may be useful for patients with a history of substance use. Dexmedetomidine can be useful for both short and longer cases, given that generally the recovery is similar to midazolam and the infusion can be maintained for the length of the procedure. The results from trials suggest it would be advantageous in more unpleasant, longer procedures such as implant surgery or sinus lifts. Although some literature suggests that this drug could be used in patients with sleep apnoea or respiratory disease, this is not supported by more recent studies. Newer evidence does not support that this drug causes less respiratory depression than other sedative agents.
Given the cardiovascular changes seen, careful patient selection is required with avoidance of patients who have pre-existing bradycardias who could go into cardiac arrest in rare cases and those with heart block. In addition, ECG monitoring is recommended and this is likely to be the largest prohibitive factor to use of the drug in dentistry because any monitoring requires interpretation and action which is out of the skill set of most dentists. As recommended in the South African guidance, the drug should only be used with a dedicated sedationist, and this guidance recommends use only in the hospital setting. It is highly likely in the UK if dexmedetomidine were to become a recognised drug in UK sedation guidance, that it would fall into the advanced technique category, and therefore require a separate sedationist. As such, the training requirements and the problems in gaining amentor for supervised cases with advanced techniques would be similar for dexmedetomidine as they are with propofol.
The drug is clearly useful in the paediatric premedication setting as an intranasal preparation and an area of research that may be interesting to explore would be intranasal dexmedetomidine compared to intranasal midazolam in adults.
Overall, the limitations of being unable to use this as an operator sedation technique and the potential significant cardiovascular changes mean that dexmedetomidine is unlikely to ever become a drug of choice for intravenous conscious sedation in dentistry.
Statement
This essay was submitted as part of the assessment for the award of a diploma in conscious sedation at King’s College London. It has not been published. It is entirely my own work.
References
1. Anttila M, Penttila J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol 2003; 56: 691-693.
2. Smuszkiewicz P, Wiczling P, Ber J et al. Pharmacokinetics of dexmedetomidine during analgosedation in ICU patients. J Pharmacokinet Pharmacodyn 2018; 45: 277-284.
3. Fan T W V, Ti L K, Islam I. Comparison of dexmedetomidine and midazolam for conscious sedation in dental surgery monitored by bispectral index. Br J Oral Maxillofac Surg 2013; 51: 428-433.
4. Makary L, Vornik V, Finn R et al. Prolonged recovery associated with dexmedetomidine when used as a sole sedative sgent in office-based oral and maxillofacial surgery procedures. J Oral Maxillofac Surg 2010; 68: 386-391.
5. Weerink M A S, Struys M M R F, Hannivoort L N, Barends C R M, Absalom A R, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet 2017; 56: 893-913.
6. Mishra N, Birmiwal K G, Pani N, Raut S, Sharma G, Rath K C. Sedation in oral and maxillofacial day care surgery: A comparative study between intravenous dexmedetomidine and midazolam. Natl J Maxillofac Surg 2016; 7: 178-185.
7. Medical Advisory Secretariat. Bispectral index monitor: an evidence based analysis. Ont Health Technol Assess Ser 2004; 4: 1-70.
8. Keating G M. Dexmedetomidine: A review of its use for sedation in the intensive care setting. Drugs 2015; 75: 1119-1130.
9. Hall J E, Uhrich T D, Barney J A, Arain S R, Ebert T J. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg 2000; 90: 699-705.
10. Cheung C W, Ng K F, Liu J, Yuen M Y, Ho M H, Irwin M G. Analgesic and sedative effects of intranasal dexmedetomidine in third molar surgery under local anaesthesia. Br J Anaesth 2011; 107: 430-437.
11. Bloor B C, Ward D S, Belleville J P, Maze M. Effects of intravenous dexmedetomidine in humans II. Anesthesiology 1992; 77: 1134-1142.
12. National Institute for Health and Care Excellence. Dexmedetomidine. British National Formulary. Online information available at https://bnf.nice.org.uk/drug/dexmedetomidine.html#cautions (accessed October 2023)
13. Belleville J P, Ward D S, Bloor B C, Maze M. Effects of intravenous dexmedetomidine in Humans I. Anesthesiology 1992; 77: 1125-1133.
14. Lodenius A, Maddison K J, Lawther B K et al. Upper airway collapsibility during dexmedetomidine and propofol sedation in healthy volunteers: A nonblinded randomized crossover study. Anesthesiology 2019; 131: 962-973.
15. Ward D S, Karan S B. Dexmedetomidine and the upper airway: Not as simple as we hoped. Anesthesiology 2019; 131: 953-954.
16. Wang L, Zhou Y, Zhang T, Huang L, Peng W. Comparison in sedative effects between dexmedetomidine and midazolam in dental implantation: A randomized clinical trial. Biomed Res Int 2020; 6130162. https://doi.org/10.1155/2020/6130162
17. Precedex® Data Sheet. New Zealand. 2023. Online information available at https://www.medsafe.govt.nz/profs/Datasheet/p/Precedexinf.pdf (accessed October 2023)
18. Takata K, Adachi Y U, Suzuki K, Obata Y, Sato S, Nishiwaki K. Dexmedetomidine- induced atrioventricular block followed by cardiac arrest during atrial pacing: a case report and review of the literature. J Anesth 2014; 28: 116-120.
19. Nagasaka Y, Machino A, Fujikake K, Kawamoto E, Wakamatsu M. Cardiac arrest induced by dexmedetomidine. Masui 2009; 58: 987 – 989.
20. Bharati S, Pal A, Biswas C, Biswas R. Incidence of cardiac arrest increases with the indiscriminate use of dexmedetomidine: A case series and review of published case reports. Acta Anaesthesiol Taiwan 2011; 49: 165-167.
21. Wood M, Wood A. Drugs and Anesthesia: Pharmacology for Anesthesiologists. 2nd ed. Baltimore: Williams and Wilkins, 1990.
22. Jakob S M, Ruokonen E, Grounds RM et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation, two randomized control trials. J Am Med Assoc 2012; 307: 1151-1160.
23. Scott-Warren V L, Sebastian J. Dexmedetomidine: its use in intensive care medicine and anaesthesia. BJA Education 2016; 16: 242-246.
24. Scoping our practice. London: National Confidential Enquiry into Patient Outcome and Death, 2004.
25. Wu W, Chen Q, Zhang L C, Chen WH. Dexmedetomidine versus midazolam for sedation in upper gastrointestinal endoscopy. J Int Med Res 2014; 42: 516-522.
26. Preskorn SH, Zeller S, Citrome L et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: A randomized clinical trial. J Am Med Assoc 2022; 327: 727-736.
27. Cheung C W, Ying C L A, Chiu W K, Wong G T C, Ng K F J. A comparison of dexmedetomidine and midazolam for sedation in third molar surgery. Anaesthesia 2007; 62: 1132-1138.
28. Üstün Y, Gündüz M, Erdogan Ö, Benlidayi M E. Dexmedetomidine versus midazolam in outpatient third molar surgery. J Oral Maxillofac Surg 2006; 64: 1353-1358.
29. Mishra N, Birmiwal K G, Pani N, Raut S, Sharma G, Rath K C. Sedation in oral and maxillofacial day care surgery: A comparative study between intravenous dexmedetomidine and midazolam. Natl J Maxillofac Surg 2016; 7: 178 – 185.
30. Nolan P J, Delgadillo J A, Youssef J M, Freeman K, Jones J L, Chehrehsa A. Dexmedetomidine provides fewer respiratory events compared with propofol and fentanyl during third molar surgery: A randomized clinical trial. J Oral Maxillofac Surg 2020; 78: 1704-1716.
31. South African Society of Anaesthesiologists. Guidelines for the safe use of procedural sedation and analgesia for diagnostic and therapeutic procedures in adults: 2020 – 2025. South Afr J Anaesth Analg 2020; 26: S1-75.
32. Keles S, Kocaturk O. Comparison of oral dexmedetomidine and midazolam for premedication and emergence delirium in children after dental procedures under general anaesthesia: a retrospective study. Drug Des Devel Ther 2018; 12: 647-653.
33. Wang L, Huang L, Zhang T, Peng W. Comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric dental patients under general anesthesia: A randomised clinical trial. Biomed Res Int 2020; 5142913. https://doi.org/10.1155/2020/5142913