Please click on the tables and figures to enlarge
Is there a differential sedative effect in ADHD patients?
N. Kafil* BDS
Foundation general dentist, Royal Free London NHS Foundation Trust, Pond Street, London, NW3 2QG
*Correspondence to: Dr Nicka Kafil
Email: drkafiln@gmail.com
Kafil N. Is there a differential sedative effect in ADHD patients? SAAD Dig. 2024: 40(II): 113-116
Abstract
Attention deficit hyperactivity disorder (ADHD) is a common but relatively undiagnosed disability which may require conscious sedation to allow dental treatment to take place. Sedation is indicated due to issues with behavioural management, however, the types of sedative agents used affect the GABAergic pathways in the brain. In studies, patients with ADHD have shown a reduced concentration of gamma-aminobutyric acid (GABA). This discussion theorises whether there is a differential impact of sedation on ADHD patients. There is a lack of literature to provide sufficient evidence to allow a conclusion highlighting a research gap in the matter, with some research contradicting others. Further studies are required to provide quantitative data to form valid conclusions. This in turn will provide recommendations to practitioners so there are evidence-based improvements in sedation for patients with ADHD.
Methodology
The methodology for the systematic literature review on dental sedation and ADHD.
The inclusion criteria consisted of:
- studies published in peer-reviewed journals
- research conducted on dental sedation, midazolam, nitrous oxide and ADHD
- studies in the English language.
Comprehensive research across PubMed databases with relevant keywords: ‘ADHD’, ‘midazolam’, ‘nitrous oxide’ and ‘conscious sedation’. The relevant publications are free full text from 1970 to 2022 with studies on humans including clinical trials, meta-analysis and systematic review. Additional reference chaining by reviewing citations of key articles.
Introduction
The American Psychiatric Association Diagnostic and Statistical Manual, Fifth Edition (DSM-5-TR) defines attention deficit hyperactivity disorder as a mental disability. It presents as a persistent pattern of inattention, hyperactivity and / or impulsivity that impacts development and executive functioning.1 These characteristics can be obstacles for dental treatment to be completed, as there is often difficulty with the inactivity required from the patient to allow treatment to be undertaken successfully. Sedation could be attempted as an adjunct to behaviour management to facilitate treatment for patients with ADHD. Midazolam is a common agent used for intravenous sedation, midazolam acts alongside gamma-aminobutyric acid (GABA) to produce the sedative effect.2 However, patients with ADHD are reported to have a reduced GABA concentration. There is limited literature about the relationship between reduced GABA in ADHD patients and the sedative effect of midazolam.3 This paper aims to discuss the current evidence and theories to determine whether sedation has a differential impact on patients with ADHD.
Sedation indications for ADHD patients
A meta-analysis estimated around 5% of children and adolescents have a formal ADHD diagnosis worldwide.1 There is a disproportionate diagnosis rate of males compared to females in childhood. This figure is an estimate either due to a lack of international acknowledgement of the validity of the DSM-5-TR or disregard of diagnosis due to stigma towards the condition. Additionally, there is ambiguity in the prevalence of ADHD in adults as the DSM-5-TR is tailored for adolescent characteristic symptoms.4 Thus, the estimated percentage is not representative of the true number of patients with ADHD due to potentially a high proportion of the population being undiagnosed.
ADHD presents as a spectrum of symptoms, impacting individuals in varying intensities which may lead to behavioural disruptions and the inability to co-operate during dental treatment. There are three subtypes of ADHD: inattentive, hyperactive, or impulsive.4 Each individual with ADHD does not fall into a specific subtype butrather on a spectrum of traits. This may cause difficulty for the dental practitioner to use behaviour management techniques to prevent distractibility and restlessness to enable treatment to be carried out safely. In these instances, sedation may be considered a viable adjunct. Other indications for conscious sedation of ADHD patients are co-morbidity of anxiety and intellectual disabilities.5
Patients with ADHD are classified as high caries risk and often require numerous dental visits. This is supported by a study in New Zealand which reported that ADHD patients were 12 times more likely to have decayed, missing and filled teeth (DMFT) than the control group.6
Blomqvist M, Ahadi S, Fernell E et al.,7 suggested that the frequency of tooth brushing for children with ADHD is 34% lower than in the control group, thus the increased number of dental visits is to allow shorter recall intervals to promote better oral health behaviours.7 Another contributing factor is the psychostimulant medications used alongside antidepressants which are typically prescribed to treat ADHD patients. These also have a common side effect of xerostomia, which contributes to the high caries risk categorisation.8
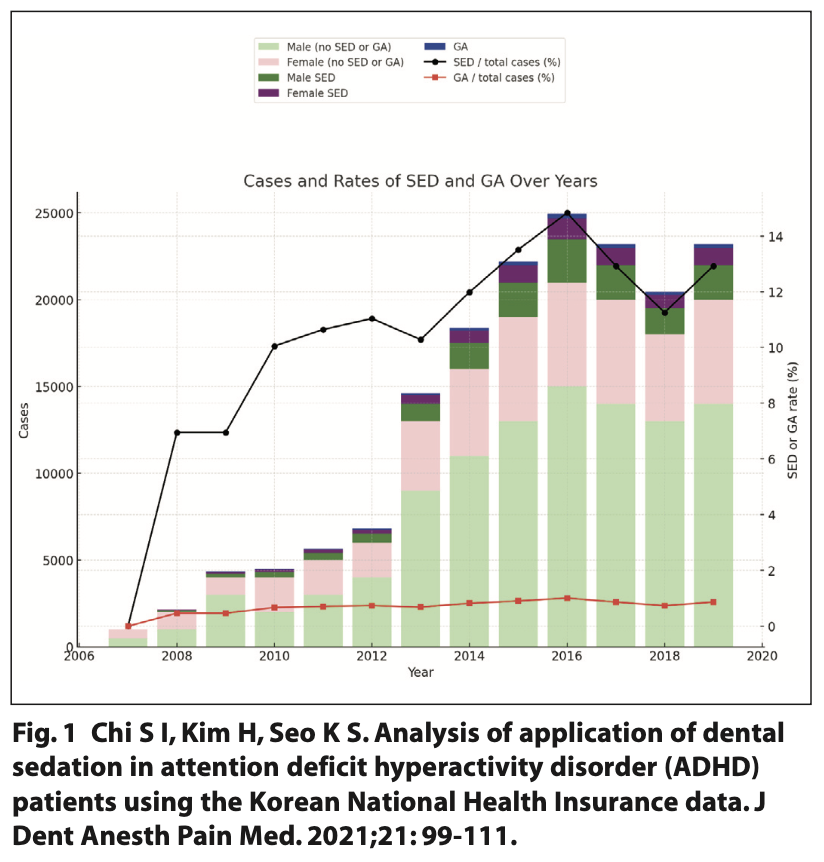
A study in South Korea investigated the yearly trend of ADHD patients undergoing dental treatment under sedation using the Korean National Health Insurance data. Figure 1 presents the annual number of ADHD patients undergoing dental treatment. It increased from 2007 to 2018 with the largest increase in sedation adjuncts being 14% in 2016. However, the number of sedation cases for ADHD patients decreased after 2016.9 Nevertheless, despite a decrease in sedation used for ADHD patients, there is still an increased need for dental treatment. This indicates that a continuation of prevention is imperative; this will aid in reducing the amount of treatment and continue to decrease the number of sedation procedures.9
Dentists have a professional and ethical responsibility to provide optimal health care for individuals with special care needs. However, in some instances, the behaviour management strategies used by dentists are insufficient for patients with ADHD. Sedation could be a useful adjunct to dental treatment for ADHD patients as it reduces the risk of complications arising during treatment due to the patient’s inability to co-operate. It also helps the patient have a less anxiety-provoking experience which may improve their perception of dental treatment, which may in turn improve their oral health in the long term. Overall, the use of dental sedation may be in the patient’s best interests.
Mechanism of action
The two most common types of conscious sedation noted in this discussion are inhalation sedation (IHS) with nitrous oxide and oxygen and intravenous sedation (IVS) with midazolam. There are other variations in administration methods and sedative agents used.
Nitrous oxide is a gas that produces effects of anxiolysis, sedation and muscular relaxation, however, it is a poor anaesthetic.10 It is characterised by rapid onset and fast recovery due to a minimum alveolar concentration (MAC) of 1.04 atmospheres absolute.11 It is administered with a mixture of oxygen to ensure the patient is respiring sufficient oxygen levels. Also, levels of nitrous oxide should not routinely exceed 50% as negative side effects are more likely to occur.10
The guidelines from the Intercollegiate Advisory Committee for Sedation in Dentistry (IACSD 2015) and the Scottish Dental Clinical Effectiveness Program (SDCEP 2017) favour nitrous oxide as a type of conscious sedation.12-13 However, it requires good co-operation with behaviour management for patients without ADHD.
Therefore, this may not be the best option for patients with ADHD as it does not overcome the issue of the complexity of the behaviour management required.
The anxiolytic impact of nitrous oxide is thought to involve the activation of GABAA receptors, although it is uncertain whether nitrous oxide acts directly or indirectly upon the binding site.14 A paper by Fujinage and Maze15 discussed the impact of nitrous oxide on opioid receptors through the release of opioid peptides. This causes the activation of nociceptive processing in descending pathways located in the spinal cord. The noradrenergic descending pathway is inhibited due to the inhibition of the GABA pathway from opioid peptide release.14
There are no current studies to conclude whether this impacts an ADHD patient’s altered GABA pathway. Inhalation sedation may not be the most optimal adjunct for ADHD patients as it still requires behaviour management. Additionally, another factor is the possible GABA-dependent activity, with unknown impacts on ADHD patients. Though it may be the preferential sedation type in guidelines, nitrous oxide may not be as suitable for ADHD patients.
Midazolam is a 1,4-imidazobenzodiazepine derivative with properties of a short duration of action, rapid onset and water solubility.16 Midazolam can be administered through multiple routes: oral, intramuscular, intranasal and intravenous. There is a rapid resorption rate due to its unique structure, with a pH-dependent ring opening which causes increased lipid solubility at a physiological pH. Midazolam is bound to plasma proteins in serum albumin with clearance of midazolam through liver metabolism by P450 cytochrome.17
Midazolam has effects of sedation, anxiolysis, temporary amnesia, muscle relaxation and anti-convulsion.2 However, there are side effects including, but not limited to, respiratory depression, excessive sleepiness, dizziness, confusion, nausea and vomiting.18
The physiological effects of midazolam are due to the action of midazolam on the receptors of the neurotransmitter GABA.2 GABAA receptors are ligand-gated ion channels that control GABA transmission, which acts as an inhibitory neurotransmitter to achieve inhibition of the central nervous system.19 Midazolam increases GABA binding to GABAA receptors, increasing the effects of GABA, thus increasing the frequency of chloride channel opening. This leads to hyperpolarisation of the membrane which decreases the action potential in the post-synaptic neurone. This causes neuronal inhibition and thus the inhibition of the central nervous system.2
GABAA receptors consist of five subunits, the gamma (γ) subunit is imperative for benzodiazepine's impact on the GABAA receptor. Benzodiazepine and GABA bind to a chloride ion channel separately. However, without the γ subunit, the benzodiazepine is unable to alter membrane potential through GABAA receptor activity.20
The association of GABA with ADHD
ADHD is a developmental disability that is associated with a deficient dopamine and noradrenergic neurotransmitter system.21 A study by the American Academy of Neurology used transcranial magnetic stimulation to present a correlation between decreased short intercortical inhibition with ADHD symptoms of reduced motor control and impulsivity. Short intercortical inhibition is modulated by GABAA agonists, which is imperative for processing sensory information and utilising appropriate behavioural responses.22 Another study using magnetic resonance spectroscopy provided evidence that also supports the hypothesis that the difficulty in behaviour inhibition is linked to reduced GABA concentration.3
Opposing literature from the New York Academy of Sciences argues that the gamma-aminobutyric acid receptor subunit theta (GABRQ) gene encoding the GABAA receptor subunit is associated with ADHD. With a variance in personal impact due to polymorphism in X-linked GABRA3 and GABRB3 genes.23 ADHD has a comorbidity with autism spectrum disorder (ASD). A study of ADHD and ASD presented that the GABRQ gene is associated with both disorders. The transgenic model noted mutations in the GABAA receptor which may be associated with reduced GABA and inhibition response. However, the GABRQ gene did not prove a significant association, suggesting a hypothesis of genetic variants across genes that produce a combined effect. Thus, additional research is necessary for clarification.24
The literature can be categorised into two main theories about the relationship between GABA and ADHD. Either there is a reduced GABA concentration or a GABAA receptor mutation in patients with ADHD. Unfortunately, none of the studies identifies whether there is reduced GABA concentration with a normal number of GABA receptors or vice versa. Additionally, the specific distribution and location of reduced GABA or GABAA receptors are not determined.3 This may be very important when considering intravenous sedation with midazolam for patients with ADHD.
Impact of sedation on ADHD patients
The impact of sedation on ADHD patients has not been studied and / or widely reported. Therefore, in this discussion the evidence in studies reporting on either theory of altered GABA released or GABAA receptor mutation is utilised to theorise the impacts.
The inhibition of neurones through GABA binding occurs in phasic and tonic forms. Phasic inhibition is controlled by synaptic receptors, resulting in the fleeting desensitisation of GABAergic conduction. Tonic inhibition occurs via extra-synaptic receptors that lead to a persistent type of GABA conductance.25 ADHD presents altered concentrations of GABA release, which causes both the phasic and tonic inhibition forms to be reduced in the amygdala; the co-ordinating centre of emotional behaviour in the brain.22
Mutation of GABAA receptor subunits leads to an ineffective response to GABA.22 Literature from Molecular Pharmacology at the University of Singapore suggests that in neurological diseases the decreased number of GABAA receptors has similar effects to the mutated GABAA receptors.27
A study on cerebral blood flow changes due to midazolam shows a dose-related reduction in regional cerebral blood flow in the cingulate gyrus, prefrontal cortex and thalamus. This causes impaired cognitive function in these areas.28 The anterior cingulate gyrus in the brain involves emotional processing and behavioural control. Additionally, it has extensive connections to the amygdala.29
From the evidence about reduced GABA concentration in the studies mentioned above it is possible to theorise that for ADHD patients there is a reduced impact from midazolam during sedation, either from decreased GABA secretion or GABAA receptor mutation. This may lead to less inhibition in the amygdala and cingulate gyrus during sedation, exhibiting reduced dampening on behaviour and emotional responses targeted in sedation. This means that whilst using midazolam, the ADHD patient is more difficult to calm and relieve their behavioural symptoms, which may interfere with dental treatment. This is also possible due to the connection between ‘emergence agitation’ and ADHD patients noted in a study by researchers at the University of Michigan.29 Emergence agitation is when patients are irrationally excited, agitated, restless and combative during early phase sedation recovery. The causative factor of emergence agitation is theorised to be due to the altered GABAA receptor activity.29 The Sachdev and Kruk model describes this as being due to decreased inhibition signals to the thalamocortical neurones, thus an increase in ADHD characteristics and less behavioural control.30
On the contrary, a study published in the Korean Journal of Anesthesiology31 reported a similar sedative effect on children with, and without, ADHD in the emergency department. The mean sedation scores recorded for each patient presented no requirements for modifications of midazolam sedation used for children with ADHD. The data presented no significant difference in sedation depth, however, there was an incidental finding of an increase in sedation duration for ADHD patients. This study had a limitation of non-standardised dosage therefore, variance could have possibly introduced differences between the cases.32 This case does not provide any input on the theory discussed previously as there are no records of whether there were any differences in the extent of ADHD symptoms presented during sedation and whether behavioural control was like patients without ADHD.
The definition and practice of conscious sedation vary between countries due to differences in health system regulations, sedation techniques and the drugs used. For instance, the maximum recommended concentration of nitrous oxide differs across European countries, ranging from 50% to 70%.33 These variations mean that success rates observed in one country cannot be directly applied to another, such as the UK. This discrepancy was highlighted in an international prospective cohort study conducted across Japan, Korea and Taiwan, emphasising that sedation practices and regulations differ globally.34 There is a limitation in the comparison of studies due to the differences in sedation practices across countries. This variation leads to challenges in assessing the impact of sedation uniformly, as different drugs and techniques may be used. This is especially important because there is very little research on sedation in patients with ADHD. The limited data available on this topic further complicates the ability to draw reliable comparisons and develop standardised guidelines for sedation practices in the ADHD patient group.
Sedation considerations for the management of ADHD patients
Patients with ADHD require both behavioural and pharmacological management to regulate cognitive abilities and behaviours. Sedation is a consideration when non-pharmacological management is not sufficient for dental treatment to occur. The use of stimulant medication is a common treatment option for ADHD, with methylphenidate (Ritalin) being the most used.6 A case report by Ririe D, Kirsten R, Navil S et al.,32 presented the difficulties of conscious sedation with oral midazolam in patients with ADHD who are medicated with Ritalin. It is a stimulant medication; therefore, it was inferred that although it decreases the behaviour impulsivity of the patient, it is potentially a reason for the difficulty in obtaining the desired sedation level. This led to increased doses and additive time required to reach the level of desired conscious sedation.32 Whilst planning for pharmacological management, the dental sedationist should consider drug interactions, as the stimulant medication could potentially counteract the sedative agents.
Another consideration is the difference in sedative drug metabolism in ADHD patients. Ritalin impacts the liver through the inhibition of microsomal enzymes which in turn impacts metabolism and elimination.8 Midazolam is metabolised in the liver via microsomal oxidase.35 It is possible to suspect that findings of prolonged sedation, in the study in the Korean Journal of Anesthesiology,31 are due to Ritalin impacting the midazolam metabolism in the patient. The dental sedationist might consult the patient’s psychiatrist before treatment. This is because there is little literature, therefore guidelines, about the interaction between ADHD stimulant medications and sedative agents.
As previously discussed, the risk of dental caries and xerostomia is higher in ADHD patients. Therefore, it is paramount for the dental practitioner to provide a tailored prevention protocol. In turn, this will reduce the number of ADHD patients who require treatment and thus sedation.
Conclusion
There are multiple case studies and theories about the correlation between ADHD and sedation. Unfortunately, there is a lack of systematic investigations with sufficient sample sizes and set variables to prove a definitive answer.
Many of the current studies available on ADHD patients are based on children, there is an overall lack of literature on the dental management of adult ADHD patients. Most patients seen by a general dental practitioner are adults, therefore further observations and different sample groups are needed to provide guidance towards sedation treatment.
Multiple quantitative studies need to be completed for adults and children with ADHD separately, each analysing the effects of dosage of sedation types compared to a control group. This should allow a meta-analysis to be conducted to provide evidence for the theories discussed. Further studies will allow specific guidelines to be formed to help the dental sedationist and provide optimum dental care for patients with ADHD.
References
1. American Psychiatric Association. 2022. What is ADHD? Online information available at: https://www.psychiatry.org/patients-families/adhd/what-is-adhd.
2. Wang J, Sun P, Liang P. Neuropsychopharmacological effects of midazolam on the human brain. Brain Informatics. 2020; Nov 10: 7-15.
3. Edden R A, Crocetti D, Zhu H et al. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69: 750-3.
4. Polanczyk G V, Willcutt E G, Salum G A et al. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43: 434-42.
5. Wilens T E, Spencer T J. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010;122: 97-109.
6. Broadbent J M, Ayers K M S, Thomson W M. Is attention-deficit hyperactivity disorder a risk factor for dental caries? A case-control study. Caries Res. 2004;38: 29-33.
7. Blomqvist M, Ahadi S, Fernell E et al. Dental caries in adolescents with attention deficit hyperactivity disorder: a population-based follow-up study. Eur J Oral Sci. 2011;119: 381-5.
8. Sinha S, Praveen P, Rani S P et al. Pedodontic Considerations in a Child with Attention Deficit Hyperactivity Disorder: Literature Review and a Case Report. Int J Clin Pediatr Dent. 2018;11: 254-259.
9. Chi S I, Kim H, Seo K S. Analysis of application of dental sedation in attention deficit hyperactivity disorder (ADHD) patients using the Korean National Health Insurance data. J Dent Anesth Pain Med. 2021;21: 99-111.
10. Hallonsten A L, Jensen B, Raadal J et al. EAPD Guidelines on Sedation in Paediatric Dentistry. 2003.
11. Röpcke H. Effects of nitrous oxide on mac. Best Practice & Research Clinical Anaesthesiology. 2001; 15: 409–16.
12. Intercollegiate Advisory Committee for Sedation (IACS). Standards for Conscious Sedation in the Provisions of Dental Care: Dental clinical guidance. 2015. Online information available at: https://www.saad.org.uk/IACSD%202020.pdf (accessed September 2023).
13. Scottish Dental Clinical Effectiveness Programme (SDCEP). Conscious sedation in Dentistry: Dental clinical guidance. 2022. Online information available at: https://www.sdcep.org.uk/media/iota3oqm/sdcep-conscious-sedation-guidance-unchanged-2022.pdf (accessed September 2023).
14. Emmanouil D E, Quock R M. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54: 9-18.
15. Fujinaga M, Maze M. Neurobiology of nitrous oxide-induced antinociceptive effects. Mol Neurobiol. 2002;25: 167-89.
16. Emmanouil D E, Quock R M. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54: 9-18.
17. Vasakova J, Duskova J, Lunackova J et al. Midazolam and its effect on vital signs and behaviour in children under conscious sedation in dentistry. Physiol Res. 2020;69: 305-314.
18. Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatric Annals. 1995;25: 158–65.
19. Wang J, Sun P, Liang P. Neuropsychopharmacological effects of midazolam on the human brain. Brain Inform. 2020;7:15.
20. Eom W, Lee J M, Park J et al. The effects of midazolam and sevoflurane on the GABA(A) receptors with alternatively spliced variants of the γ2 subunit. Korean J Anesthesiol. 2011;60: 109-18.
21. Kim Y S, Yoon B E. Altered GABAergic Signalling in Brain Disease at Various Stages of Life. Exp Neurobiol. 2017;26: 122-131.
22. Gilbert D L, Isaacs K M, Augusta M et al. Motor cortex inhibition: a marker of ADHD behaviour and motor development in children. Neurology. 2011;76: 615-21.
23. Rothman D L, Petroff O A, Behar KL et al. Localised 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90: 5662-6.
24. Comings D E. Clinical and molecular genetics of ADHD and Tourette syndrome. Two related polygenic disorders. Ann N Y Acad Sci. 2001;931: 50-83.
25. Naaijen J, Bralten J, Faraone S et al. Glutamatergic and GABAergic gene sets in attention-deficit/hyperactivity disorder: association to overlapping traits in ADHD and autism. Transl Psychiatry. 2017;7:999.
26. Lee V, Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits. 2014;8:3.
27. Yuan H, Low C-M, Moody O A et al. Ionotropic GABA and Glutamate Receptor Mutations and Human Neurologic Diseases. Mol Pharmacol. 2015;88: 203-17.
28. Powers W J. Cerebral blood flow measurement with positron emission tomography. Cerebral Blood Flow. 2003: 217–25.
29. Rolls E T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. 2019;224: 3001-3018.
28. Reddy S K, Deutsch N. Behavioral and Emotional Disorders in Children and Their Anesthetic Implications. Children (Basel). 2020;7: 253.
29. Lindenmayer J P. The pathophysiology of agitation. J Clin Psychiatry. 2000;61: 5-10.
30. Catharine A W, Dawn E, Trevor R. Behavioural models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Clin Psychol Rev. 2006; 26: 379–395.
31. Shin Y H, Kim M H, Lee J J et al. The effect of midazolam dose and age on the paradoxical midazolam reaction in Korean paediatric patients. Korean J Anesthesiol. 2013;65: 9-13.
32. Ririe D, Kirsten R, Navil S et al. Unexpected interaction of methylphenidate (Ritalin) with anaesthetic agents. Paediatr Anaesth. 1997;7: 69-72.
33. Ashley P, Anand P, Andersson K. Best clinical practice guidance for conscious sedation of children undergoing dental treatment: an EAPD policy document. European Archives of Paediatric Dentistry. 2021; 22: 989–1002.
34. Yang C H, Chen P J, Mori M et al. Cross-cultural comparison of continuous deep sedation for advanced cancer patients in East Asian countries: prospective cohort study. Japanese Journal of Clinical Oncology. 2023; 1–9.
35. Brichard G, Johnstone M. The effect of methylphenidate (Ritalin) on post- halothane muscular spasticity. Br J Anaesth. 1970;42: 718-22.